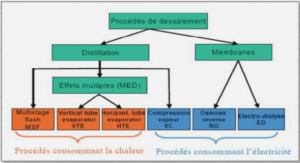
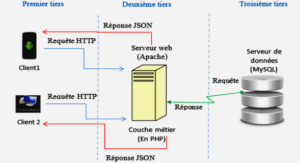
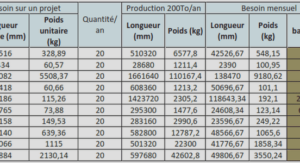
Conversion of bicarbonate into biomass and calcium carbonate by the Cyanobacteria strain Synechococcus sp. PCC 8806
Utilization of bicarbonate in cyanobacterial photosynthesis releases hydroxyl ions which react with bicarbonate to produce carbonate. In sea water this increase in carbonate concentration can lead to calcite precipitation in the presence of calcium. In natural environments, this could take place in conditions such as high density cell blooms or mats. Cyanobacteria considered as calcifiers obviously rely on this physiological mechanism to perform calcification and photosynthesis in a putative 1:1 molar ratio. Our study was designed to thoroughly investigate this photosynthesis/precipitation ratio. We used the Rock Eval® technology to determine the quantity of both carbon and calcium incorporated into the biomass and calcium carbonate. Synechococcus PCC 8806 was grown in mineral culture medium enriched with various bicarbonate and calcium concentrations in a closed reactor preventing atmospheric CO2 Cyanobacteria have been reported as biological contributors for high oversaturation in carbonate of their microenvironment (Thompson et al., 1997). All the proposed cyanobacterial precipitation mechanisms are commonly based on mineral carbonate precipitation reactions linked to cellular physiological processes, according to the in vivo cyanobacterial CO2 assimilation model driven by CCM enhanced photosynthesis (Miller & Colman, 1980; Thompson & Ferris, 1990; Kaplan et al., 1991; Merz, 1992; McConnaughey, 1994; Price et al., 1998; Kaplan & Reinhold, 1999; Badger & Price, 2003). The microscopic observations of organisms embedded in non structured calcite crystals show that the growth of crystals probably happens in the microenvironment of cell outer membranes (Dittrich and Obst 2004).
calcification deposition events. Several field studies have stimulated the question on biologically active precipitation with cyanobacteria (Morse et al., 2003). Whiting settings have been extensively studied: for instance the Fayetteville Green Lake (hard water lake) (Thompson and Ferris, 1990; Thompson 1997) and the Great Bahamas Bank (marine environment) (Thompson, 2000)… Sedimentation rates of carbonate precipitates have been estimated from field and experimental data. In Fayetteville Green Lake (FGL), Thomson et al. (1997), on site, measured calcite precipitations between 3.5-4.0 mg per litre in the open water column between the water surface and 2 m below the surface during the spring, and even greater concentrations during the summer with a depth of 8 m. These authors also measured a decrease of the Dissolved Inorganic Carbon (DIC) concentration and inversely an increase of Moreover no studies present clearly reliable mass balance for carbon and calcium, the major elements involved in cyanobacterial calcification processes. Merz’s pioneer work (1992) on microbial mats has reported an approximate 1:1 ratio of calcification to photosynthesis (around 1 to 1.2 mol carbon fixed in biomass for 1 mol precipitated carbonate) under field conditions. Nevertheless the biomass was not measured directly. Daily variations of alkalinity and calcium have been observed in the interstitial water of the mats in the reported experiments. These variations have been used to indirectly calculate the carbon incorporated in calcium carbonate precipitates. Yates and Robbins (1998) also calculated calcification from initial and post incubation alkalinity, pH, and calcium measurements to determine the ratio between calcification and photosynthesis. These authors mentioned that DIC calculation based on alkalinity and pH variations may not accurately reflect calcification or photosynthesis.
precipitation by cyanobacteria, it is crucial to have accurate mass balances of carbon and calcium during the growth. Mass balances are difficult to obtain because the calcified cyanobacteria are a mix of organic and inorganic carbon which is ill-suited for most analytical methods. The purpose of this study was to develop and validate an analytical strategy based on the use of the Rock-Eval 6 (RE6) technique to determine the carbon distribution in calcium carbonate and biomass during cyanobacterial growth on bicarbonate. RE6 is able to determine precisely the organic and inorganic carbon of rocks (Behar et al., 2001), but has never been applied to the quantitative and qualitative characterisation of biological samples thus far. In this study, the RE6 was calibrated with pure cyanobacterial biomass cultivated in calcium deprived medium and pure calcium carbonate. It was utilized for the monitoring of bicarbonate assimilation and CaCO3 precipitation by the marine calcifying cyanobacterium..