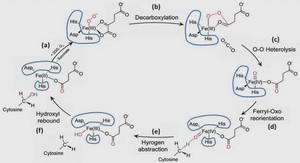
Thermodynamics of Metal Hydrides
Metal hydrides are generally formed by simply exposing the metal to hydrogen gas. A reversible reaction ofhydrogen with metal, forming metal hydride can be written as X Msolid + 2″ H2 gas ~ MHx solid + Heat,.1H = enthalpy of formation < 0 Where M is the metal element or alloy, MHx is the metal hydride and .1H is the enthalpy of formation of the metal hydride. Here, forward reaction is exothermic (~H < 0), that means heat is released, whereas the reverse reaction is endothermic (~H > 0), there is a need to add heat to release the hydrogen. The reaction of the metal or alloy with hydrogen gas is illustrated in Figure 1.1 by pressure -composition – temperature curve (peT curve). When at a given temperature, hydrogen gas is introduced into a vessel containing metal or alloy, fIrstly, hydrogen molecule dissociates into hydrogen atom on the material surface. Then hydrogen atom dissolves in crystallattice of the metal or alloy to form a solid solution (designated as the aphase). Further increase in pressure ofhydrogen, leads to more dissolved hydrogen.
When saturation limit of the solid solution phase is reached, the metal hydride begins to form as a distinct second solid phase (designated as ~-phase). Pressure of the hydrogen remains constant and hydrogen to metal ratio increases with progressive conversion of a-phase into ~-phase. This pressure is known as plateau pressure. The plateau continues, as long as there are two distinct phases, as required by Gibbs ‘ phase mIe: F=C – P + 2 But temperature is fIxed, so F = C – P + l Where F is the degree of freedom, P is the number of phases and C is the number of components. In plateau pressure region, C = 2 (Hydrogen gas and metal), P = 2 (a-phase and ~-phase) , so F = l The plateau region is the most important segment ofthe PCT curve. The value ofplateau pressure represents the pressure of hydrogen in equilibrium with the metal-metal hydride (a + ~) phases, or, also known as dissociation pressure of the metal hydride at a certain temperature. Its value indicates the thermal stability of the metal hydride and can be used as an index of the relative thermal stabilities of different metal hydrides at a given temperature.
ABs-type alloys
ABs-type alloys are mainly combination of rare earth metals and d-block metals. Generally, ABs compounds form hydrides at equilibrium pressure of a few atmospheres and at temperature up to 100 oC [11]. These alloys have good hydrogen storage capacity (~ 1.5 wt%), low hysteresis, resistance to the gaseous impurities and easy activation in the initial cycle. These alloys have a hexagonal structure with CaCus type lattice. The most studied alloy in this group is LaNis, which reacts with hydrogen as follows: However, nickel and lanthanum are the main metals in LaNis which makes it costly. Upon hydrogenation, the crystal structure remains hexagonal but with a lattice expansion of about 25%. Here, La has strong affmity for hydrogen and Ni does not absorb hydrogen or absorb little. However, the major issues with LaNis alloy are high cost of raw material and low theoretical hydrogen capacity (1.5 wt%). Several research work on tackling these two major issues ofLaNis alloy has been reported in literature [12-16]. Partial substitution of Ni changes the thermodynamic properties and also reduces the degradation during pressure cyc1ing [Il]. Effect of partial substitution of Ni with Al, Cr, Cu, Sn, Fe, Mn, Co, Ga, Zn or other metals has been investigated by Sharma et al. and the y found that partial substitution of Ni with Al, Co and Mn provides a wide range of thermodynamic properties [14].
Sharma et al. also found that partial substitution of Ni with Al improves cycling stability and also reduces the plateau pressures significantly. Partial substitution of La with Ce in LaNis was also investigated in order to improve its hydrogen storage capacity and plateau pressures for high pressure applications [16]. However, CeNis requires several activation cycles under high hydrogen pressure before frrst absorption in order to absorb hydrogen [17]. Jain et al. [18] studied the effect of partial substitution of Ni with Cr in CeNis on the hydrogen storage properties of CeNis alloy and results reveals that maximum hydrogen storage capacity of substituted alloys was higher than parent alloy (CeNis) with a value of3.5 and 3.8 for CeNi4Cr, CeNbCr2, respectively. Partial substitution of Ce with La in CeNÏ)Cr2 was also investigated by Jain et al. [19] as a continuation ofprevious study [18] and they found that increase in La content improves the maximum hydrogen storage capacity while decreases the plateau pressure. Partial substitution of Ni with Zr in CeNis was also performed by Jain et al. [20] and results clearly indicates that maximum hydrogen capacity was around 4 and 4.3 for the CeNi4Zr and CeNbZr2 alloy, respectively at operating tempe rature (293- 333 K) under 3.2 MPa pressure conditions.
AB2-type (Lave phase) alloys
In AB2-type alloys, A can be one or more oftitanium, zirconium, hafnium and rare earth elements and B can be one or more of vanadium, chromium, manganese and iron. It consists of three structure type which are cubic MgCU2 (CI5), hexagonal MgZn2 (CI4) and hexagonal MgNh (C36). This family of structure types is known as Laves phase. Laves phase makes a large group ofalloys, therefore, it has wide range ofproperties. In AB2 alloys, mostly, Zr based alloys has been studied previously due to their good hydrogen storage capacity (- 2 wt%) and kinetics, long cycling life and low cost [21]. However, these alloys are more sensitive to the gaseous impurities and their hydrides are too stable at room temperature. Multicomponent systems Zrl -xTx(Mn, Cr)2-yMy where T = Ti, Y, Hf, Sc, Nb and M = V, Mo, Mn, Cr, Fe, Co, Ni, Cu, Al, Si, Ge is an effective way to optimize the hydrogenation properties of AB2-type alloys [17, 22-25]. A systematic investigation on the hydrogen storage properties ofvanadium (V)-containing or V-free AB2-type alloys has been shown by Young et al. [24]. They observed that hydrogen st orage capacity of V -free (Til oZr27NbsCosMnl sCrsAlo. l) alloy was larger in gaseous phase compared to the V-based alloy (TiI 2Zr21.SYIO Nb7.7Mn13.sCr4.sAlo.sSno.3 ). Partial substitution with other transition metals in ZrFel.sMo.2 alloy where M = Y, Cr, Mn, Fe, Co, Ni, Cu, Mo was studied by Mitrokhin et al. [17] in order to improve the hydrogenation properties and they found the larger hydrogen storage capacity – 1.8 wt% for Y, Cr and Mn substitution than other substituted alloys. Young et al. also investigated the effect of cobalt (Co) with yttrium (Y) on the hydrogen storage performance of Zr2I.sYIOCf7.sMns. lCos.oNb2.2Sno.3Alo.4 alloys [25] and they found that maximum reversible hydrogen storage capacity (1.36 wt%) could be obtained at 333 K for the 2 at % Y. These previous studies on AB2 alloys suggest improvement in the hydrogen storage capacity and that the alloys are more attractive for stationary applications.
Solid solution alloys Ti-based Laves phase alloys of AB2-type contains the body-centered cubic (BCC) and Laves phases, where both phases contribute to the hydrogenation. These phases show the same equilibrium pressure. Due to this reason, this new class of alloys has been called Laves phase related solid solution alloys. In solid solution alloys, vanadium-based solid solution alloys with body-centered cubic (BCC) structures have been extensively studied because of their good hydrogen storage capacity (- 4 wt%), low hydrogen absorptionldesorption temperature and fast frrst hydrogenation kinetics. However, these alloys are still considered to be not satisfactory for vehicular application due to the involvement of activation step before frrst hydrogenation. Several methods have been applied earlier to the BCC solid solution alloys in order to improve the frrst hydrogenation kinetics such as annealing [26], surface modification [27], element substitution [28] . Young et al. studied the effect of annealing on Laves phase related BCC (body-centeredcubic) solid solution metal hydride alloys [26]. They found that annealing at 800 0 C and 900 0 C promotes the formation of the main hydrogen storage BCC phase abundance and reduced C14 and TiNi catalytic phases. Ti-based solid solution alloys with BCC structure such as Ti- Y-Cr, Ti- YMn and Ti-Cr- Mo were also studied previously because it has good hydrogen capacity – 2 wt% and fast absorption kinetics [29, 30). These Ti-based alloys su ch as Tio.s Yo.sMn has fast frrst absorptionldesorption kinetics and high reversible hydrogen capacity – 1.9 wt% at 260 K temperature under 35 MPa [31). Ali these previous investigations on BCC solid solution make possible hydrogen absorption at reasonable temperature and pressure and suggest that these can be potential hydrogen storage material.
ABs-type alloys ABs-type alloys are mainly combination of rare earth metals and d-block metals. Generally, ABs compounds form hydrides at equilibrium pressure of a few atmospheres and at temperature up to 100 oC [11]. These alloys have good hydrogen storage capacity (~ 1.5 wt%), low hysteresis, resistance to the gaseous impurities and easy activation in the initial cycle. These alloys have a hexagonal structure with CaCus type lattice. The most studied alloy in this group is LaNis, which reacts with hydrogen as follows: However, nickel and lanthanum are the main metals in LaNis which makes it costly. Upon hydrogenation, the crystal structure remains hexagonal but with a lattice expansion of about 25%. Here, La has strong affmity for hydrogen and Ni does not absorb hydrogen or absorb little. However, the major issues with LaNis alloy are high cost of raw material and low theoretical hydrogen capacity (1.5 wt%). Several research work on tackling these two major issues ofLaNis alloy has been reported in literature [12-16]. Partial substitution of Ni changes the thermodynamic properties and also reduces the degradation during pressure cyc1ing [Il]. Effect of partial substitution of Ni with Al, Cr, Cu, Sn, Fe, Mn, Co, Ga, Zn or other metals has been investigated by Sharma et al. and the y found that partial substitution of Ni with Al, Co and Mn provides a wide range of thermodynamic properties [14].
Sharma et al. also found that partial substitution of Ni with Al improves cycling stability and also reduces the plateau pressures significantly. Partial substitution of La with Ce in LaNis was also investigated in order to improve its hydrogen storage capacity and plateau pressures for high pressure applications [16]. However, CeNis requires several activation cycles under high hydrogen pressure before frrst absorption in order to absorb hydrogen [17]. Jain et al. [18] studied the effect of partial substitution of Ni with Cr in CeNis on the hydrogen storage properties of CeNis alloy and results reveals that maximum hydrogen storage capacity of substituted alloys was higher than parent alloy (CeNis) with a value of3.5 and 3.8 for CeNi4Cr, CeNbCr2, respectively. Partial substitution of Ce with La in CeNÏ)Cr2 was also investigated by Jain et al. [19] as a continuation ofprevious study [18] and they found that increase in La content improves the maximum hydrogen storage capacity while decreases the plateau pressure. Partial substitution of Ni with Zr in CeNis was also performed by Jain et al. [20] and results clearly indicates that maximum hydrogen capacity was around 4 and 4.3 for the CeNi4Zr and CeNbZr2 alloy, respectively at operating tempe rature (293- 333 K) under 3.2 MPa pressure conditions.
Chapter1 |