Le système de surveillance français des
salmonelles
Salmonella est un pathogène zoonotique alimentaire qui fait, en France, l’objet d’une volonté de lutte intégrée de longue date, soutenue par le cadre réglementaire européen et s’appuyant sur un système de surveillance exemplaire, couvrant l’ensemble de la chaîne alimentaire, de la fourche à la fourchette. Le système de surveillance intégré des salmonelles en France repose à la fois sur des réseaux de surveillance passive (remontant à 1947 pour les cas humains et à la fin des années 80 pour les principaux réservoirs animaux) et sur un système de surveillance active qui a évolué de façon importante sur les 15 dernières années, sous l’influence de la réglementation européenne. L’article suivant présente l’architecture du système de surveillance français, ses acteurs et les données produites. Pour chaque secteur, secteur humain puis secteurs vétérinaire et agroalimentaire, le contexte réglementaire européen est d’abord rappelé, puis l’organisation nationale de la surveillance est mise en lumière. La surveillance pour les secteurs vétérinaire et agro-alimentaire a été considérée en trois temps, surveillance des animaux de rente, surveillance des denrées alimentaires et surveillance de l’antibiorésistance. Chaque dispositif, qu’il soit actif ou passif, institutionnel ou non, a fait l’objet d’une analyse détaillée selon les critères de l’OIE (Office International des Epizootie) et de l’EFSA (European Food Safety Agency) concernant l’évaluation des systèmes de surveillance. Enfin, les forces et faiblesse du système, sa dynamique et sa capacité à répondre aux objectifs usuels de la surveillance sont discutées. Le manuscrit présenté a fait l’objet d’une soumission à Zoonoses and Public Health et est en cours de révision.
Architecture of the Salmonella surveillance system
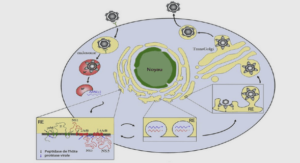
Salmonella, as a zoonotic pathogen, is the subject of several European regulations. This chapter presents the European regulatory framework and the organization of the national surveillance system, from human cases to animal sources. The actors involved in this system have previously been described by Dufour and La Vieille (Dufour and La Vieille 2000) (Figure 1).
Human sector
European regulatory framework
The surveillance of sporadic salmonellosis (i.e. not associated with an outbreak) is not regulated at European level. However, surveillance of communicable diseases based on networking of expertise in the EU Member States is promoted by Commission Decision 2119/98/EC. In 2005, under the Zoonosis Directive (Directive 2003/99/EC), the investigation and reporting of foodborne outbreaks became mandatory (Gervelmeyer, Hempen et al. 2008). At the same time, the European Centre for Disease Prevention and Control (ECDC) was established (Regulation (EC) no. 851/2004) in order to enhance the capacity of the Community and the Member states to protect human health through the prevention and control of human diseases. As far as reporting is concerned, EFSA and ECDC jointly analyze all data from the human and agro-food sectors. The results are published in an annual Community Report (2009).
National surveillance system (table 1)
The declaration of foodborne outbreaks has been mandatory in France since 1952 (Decree no. 52-953 of 7 August 1952), allowing outbreak cases surveillance. Foodborne outbreaks are defined as a group of at least two cases with mostly digestive symptoms that can be linked to a common food source. An outbreak will be confirmed as caused by Salmonella, if a Salmonella strain is isolated from a sample coming either from one of the patients or from the suspected food item. Outbreaks must be notified to either the official “départemental” (“départements” are subdivisions of the national territory administered by a Prefect) sanitary or veterinary services (named DDASS or DDSV respectively). InVS centralizes those data at national level. The DDSV and DDASS databases are merged and 62 duplicate data are eliminated. In 2007, data on 1,436 foodborne outbreak cases associated with Salmonella were centralized. For some outbreaks, specific epidemiological investigations are performed, using data collected during further enquiries and data provided by the National Reference Centre (NRC) and the Salmonella Network for the food data (described below in § 1.2.2.2) (Espié, Weill et al. 2005a; Espié, De Valk et al. 2005b; Dominguez, Jourdan-Da Silva et al. 2008). Descriptive results and epidemiological studies are published in the weekly epidemiological bulletin (Bulletin épidémiologique hebdomadaire, http://www.invs.sante.fr/BEH/) and in scientific publications (Vaillant and Espie 2005; Brouard, Espié et al. 2007; Jourdan, Le Hello et al. 2008). No mandatory notification is required for sporadic salmonellosis cases. However, passive laboratorybased surveillance of Salmonella detection from human samples, carried out by NRC since 1947, allows the collection of data on sporadic cases. This surveillance system relies on a stable network of voluntary clinical laboratories (private or hospital-based) representing 30 to 40% of all French clinical laboratories involved in human medicine. Laboratories send NRC either strains of Salmonella or reports on the strains isolated and serotyped on a daily basis. Strains and reports are registered together with epidemiological information such as travel, age and sex of the patient, type of sample taken (stool, blood…) or geographic location. A biological specimen bank and a national database on human strains are constituted. NRC registers data on about around 10,000 cases a year. Statistical analyses are performed on a weekly basis to detect unusual events, and all surveillance data are communicated to InVS. Data and analysis are published in the annual activity report of the NRC (http://www.pasteur.fr/sante/clre/cadrecnr/salmcnr/salmcnr-actualites.html) and through scientific publications(Weill, Guesnier et al. 2006).
Agro-food sector
European regulatory framework
Salmonella in food animals The first step in Salmonella surveillance in food animals at European level was the implementation of Council Directive 1992/117/EC. This Directive concerned measures for protection against specified zoonoses and zoonotic agents, in animals and products of animal origin, in order to prevent outbreaks of foodborne infections. One goal of Directive 1992/117/EC concerned the control of Salmonella in the 63 Gallus gallus species, i.e. broilers and laying hens, focusing especially on the eradication of the serotypes Enteritidis and Typhimurium in breeding animals. In 2003, this Directive was replaced by Directive 2003/99/EC of the Council and Parliament and Regulation (EC) no. 2160/2003. Directive 2003/99/EC on the monitoring of zoonoses and zoonotic agents aims to improve and coordinate the monitoring of zoonotic agents in the Community and to collect data that is easier to compile and compare. This should enable hazard identification and characterization, and exposure assessment related to zoonotic agents. Salmonella and its antimicrobial resistance are covered by this harmonized monitoring. Regulation (EC) no. 2160/2003 describes the progressive and proportionate organization of the control of Salmonella and other specified foodborne zoonotic agents at European level. Member States must implement programs to reduce the prevalence of Salmonella in farm animals and products of animal origin. Poultry (broilers, laying hens and turkeys) and pigs, considered as the major animal reservoirs, are the first productions concerned by these control measures at breeding and production level. Within this framework, harmonized prevalence studies, so-called “baseline studies”, have been conducted since 2004, to obtain scientifically relevant data about the initial level of prevalence in each Member State. On the basis of these results, mitigation targets are set species by species for specified serotypes. For example, a maximal prevalence of 1% must be met for the serotypes Hadar, Infantis, Virchow, Enteritidis and Typhimurium by the end of 2009 in breeding flocks of laying hens and broilers (Regulation (EC) no. 1003/2005).
Salmonella in food In 2002 the European
Parliament adopted the “Food Law”, (Regulation (EC) no. 178/2002), laying down the general framework to ensure a coherent approach in the development of food legislation from farm to table; EFSA was created as part of this framework. The “Food Law” establishes principles and responsibilities, the means of providing a strong scientific base, efficient organizational arrangements and procedures to underpin decision-making in matters of food and feed safety. The principle of transparency for the consumer is also established. A package of three regulations and one directive constitutes the food hygiene legislation dedicated to food business operators (FBO) and is completed by two other regulations relating to official controls and feed hygiene. General rules for FBOs, including primary production, are laid down by Regulation (EC) no. 852/2004. FBOs must ensure that their products satisfy the hygiene requirements set by Regulation (EC) no. 64 2073/2005 laying down the microbiological criteria and the implementation of hygienic rules. One of the criteria set is the absence of Salmonella in the product for safety criteria (i.e. defining the acceptability of the product) and at specific stages of the process for process hygiene criteria (i.e. setting an indicative contamination value above which corrective actions are required). This is to be met according to HACCP (Hazard Analysis Critical Control Points) principles and be scientifically justified. To ensure verification of compliance with the “Food Law”, official controls are performed according to Regulation (EC) no. 882/2004. They must be planned on the basis of risk assessment, relying on a scientific approach, in order to obtain an objective selection of products and operators to be controlled.
Antimicrobial resistance in food animals
Since the end of the nineties, several international scientific reports and recommendations have led to publications recommending the harmonization of surveillance and the regulation of foodborne antimicrobial resistance and the use of antimicrobials in animals, based on public health concerns (WHO, 1997 and 1998; Copenhagen, 1998; FAO/WHO/OIE 2003 and 2007, Codex 2005, OIE 2006). Consequently, a European strategy against the microbial threat has been defined in agreement with the “precautionary principle”. The use of antimicrobials as growth promoters in animal productions has been banned (Council Regulations (EC) no. 2821/98 and (EC) no. 2788/98). At the same time, surveillance systems for non-human use of antimicrobials and for antimicrobial resistance have been implemented at all levels of the food chain and a Community Reference Laboratory has been nominated and financed to promote harmonization of the methods used to assess antimicrobial resistance. For Salmonella, a harmonized continuous monitoring system in food animals is being implemented on the basis of a selection of strains isolated during the mandatory control programs (Decision 2007/407/EC). National data on antimicrobial resistance have had to be declared to and have been published by EFSA since 2003 (2009).
National surveillance system
The national surveillance system for Salmonella in the agro-food sector relies on active institutional surveillance coordinated by the French Food Directorate (DGAL) operating under the European regulatory framework, completed by a passive system based on networks centralizing non-human 65 strains of Salmonella from public and private veterinary laboratories. Both active and passive surveillance systems are presented following.
Active surveillance system
Salmonella in food animals (table 2)
Based on a pre-existing voluntary program, the continuous mandatory monitoring and control plan in breeding flocks of Gallus gallus has been implemented in France since 1999 in accordance with Directive 1992/117/EC. The serotypes Enteritidis and Typhimurium were targeted in breeding flocks of broilers and laying hens until 2007. Since 2007, this plan has evolved in line with Regulation (EC) 2160/2003 and its implementing regulations concerning the control of Salmonella. It is in the process of being widened to include turkeys and pigs, all species being monitored at breeding and production level, and is still targeted on the serotypes Enteritidis and Typhimurium. In Gallus gallus breeding flocks, 3 more serotypes are regulated: Hadar, Infantis and Virchow. These regulated serotypes are defined as notifiable disease submitted to sanitary policy measures (Rural Code, article D223-21). However, information on all the serotypes will be made available thanks to the classification of all Salmonella serotypes as notifiable diseases not subject to sanitary policy measures (Rural Code, article D223-1) for the regulated animal sectors. The continuous monitoring program concerns all flocks and herds and is thus exhaustive for the regulated sectors. This should lead to an annual database giving information on the national prevalence of the different Salmonella serotypes and their evolution in the regulated animal species at farm level. To prepare the enforcement of these monitoring and control programs, the initial level of prevalence of Salmonella spp has been evaluated through baseline studies between 2004 and 2009. Sampling plans have been designed to assess a prevalence of 20 to 50 % (according to the species) with a precision of 3 to 5%. They have been conducted under the responsibility of DGAL, by national and departmental veterinary services in collaboration with the National Reference Laboratory (NRL) for Salmonella.