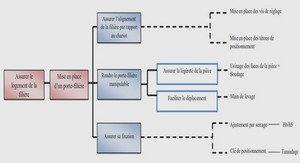
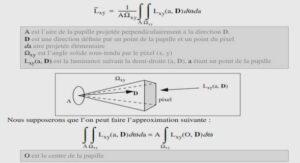
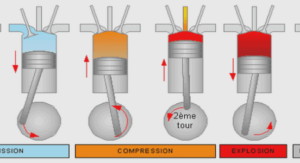
In laser physics, particularly in photonics, new materials with improved characteristics are required. In the list of commonly used materials leading position occupy the oxide crystals among which especially highlight the multifunctional crystals with optical, nonlinear optical and other properties. Among these crystals, Lithium Niobate (LiNbO3, LN) is one of the mainly used due to its various applications in optics and quantum electronics.
Lithium Niobate shows one remarkable property, namely photorefractivity, which appears under the illumination with visible or near-infrared light and leads to semi-permanent changes in the index of refraction of the crystal. As known, the dominant mechanism of the photorefraction in LiNbO3 is the bulk photovoltaic effect. Therefore, the photorefractive properties of LiNbO3 in the first instance are defined by the presence of photovoltaic-active ions, belonging to multi-charge transition metal impurities, mainly Fe, as well as Cu, Mn, Ni, etc.
By contrast, the photorefractive property is the main drawback for electro-optics and frequency conversion applications since it causes beam distortion and “optical damage”. Therefore many studies concern the resistance to photorefraction by adding to LN an appropriate dopant, such as MgO (at concentrations of 5-6 mol%).
Raman spectroscopy is a powerful technique which provides a number of information about physical or chemical properties of a material. It can give information about the structure defects of the lattice as well. That’s why it was interesting to study how the properties of LN can reflect on Raman spectra.
The aim of my study is Raman studies in photorefractive LN and by this way to investigate the peculiarities and consequences of the photorefractive properties on the Raman spectra. Several mechanisms are associated with the photorefractive effect. The question is how these phenomena affect the Raman spectra. We thus need to distinguish the successive consequences of the laser beam input: thermal change, the space charge field, and since LN is piezoelectric the subsequent lattice strain. Therefore we can speak about photo-electrostriction effect. In a complement we study the Zr doping on LN which is a candidate for inhibiting the photorefractive effect.
Lithium Niobate (LiNbO3, LN) [1] belonging to the ABO3-type ferroelectrics with oxygen octahedra, is a material that does not exist in nature, but since their first synthesis in 1949, they have been intensely studied [2,3]. Lithium Niobate is a key material in photonic technology, characterized by a combination of several favorable properties, such as high nonlinear optical coefficients, wide transparency, established technology for fabricating waveguides, and a periodically poled structures. It is widely used in various applications such as electro-optics, acousto-optics and frequency conversion. LN shows another remarkable property, namely photorefractivity (sometimes called optical damage), which appears under the illumination with visible or near-infrared light and leads to semi-permanent changes in the index of refraction of the crystal. This leads to beam distortion and a drastic decrease of the device efficiency.
Another very interesting property of the crystal is the bulk photovoltaic effect. A feature expressed by some impurities in the material resulting in the generation of photo-induced currents or voltages under certain light incidence. The structure of LiNbO3 at room temperature (RT) belongs to the rhombohedral (trigonal) space group R3c, with point group 3m. Above the phase transition temperature (1150°C) the crystal transforms into the centrosymmetric space group R3m. The setting of the crystallographic axes for the trigonal symmetry is not monosemantic. As discussed in the following section there are three choices of axes for LiNbO3, namely, rhombohedral, hexagonal, and orthohexagonal cells. For the structure description or determination as well as for crystallographic aims rhombohedral and hexagonal settings are more suitable. For most applications the orthohexagonal setting, where all axes are mutually orthogonal, is preferable. The resistance to optical damage can be increased considerably by changing the lithium niobate (LN) composition from congruent (cLN; [Li]/[Nb]=0.94) to stoichiometric (sLN: [Li]/[Nb]=1) or by adding to LN an appropriate dopant. Among the many dopants that have been tested, the most utilized is, at present, MgO that is known to be effective in molar concentrations above 5 mol% . The main problem is that it is very difficult to grow large homogeneous Mg doped LN crystals. Crystals with alternative non-photorefractive ions were recently synthesized with tetravalent ions (Hf4+, Zr4+, etc), which were found to be very efficient with an optical damage threshold around – 2-3mol%.
Introduction |