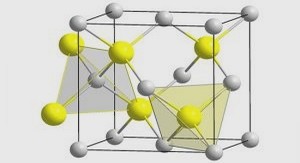
Etude des propriétés thermodynamiques des nouveaux fluides frigorigènes
Experimental techniques review
Before describing the equipment used for our experiments, it is of interest to give a brief review about the existing experimental techniques used for phase equilibria, and the classifications used in general to design them. An example of the classifications is given in Figure 2.1, for high-pressure phase equilibria techniques. According to this classification (Dohrn et al. 2010), the different phase equilibria techniques are ranked under two main methods: “Analytical methods” and “Synthetic methods”. Figure 2.1 Experimental methods classifications for high-pressure phase equilibria (Dohrn et al. 2010)
Analytical methods
For the “analytical methods”, we usually work without knowing the overall mixture composition, and through the method, we determine “analyse” the composition of the phases, hence the designation “analytical methods”. Under this category, different techniques can be found, classified under two main classes: “with sampling” and “without sampling”. For the “analytical methods with sampling”, the phase composition analysis is carried out not under pressure. Under this category, we find several techniques such as the “isothermal techniques” in which the measure is achieved at fixed temperature; the “isobaric techniques” where the working pressure is fixed (an example of these techniques is the ebulliometer with sampling) and the “isobaric-isothermal” techniques. For the “analytical methods without sampling”, the analysis of the phase composition is performed under high pressure. Under this category, different techniques can be found, like the “spectroscopic techniques”, and the “gravimetric techniques” (e.g. the suspension balances). The full details and description of these different techniques can be found in (Dohrn et al. 2010; Fonseca et al. 2011) papers.
Synthetic methods
For the synthetic methods, the overall mixture composition is exactly known in advance, and no analysis of the equilibrium phase is performed. The synthetic methods can mainly be classified under two main families: “methods with a phase change” and “methods without a phase change”. For the “synthetic methods with a phase change”, the detection of the phase change can be “visual”, like when using a static view cell, or “non-visual”, such as determining the phase change from the slope of the P-V curves. Under the “synthetic methods without a phase change” category, we find “isothermal techniques”, such as static PTx method, allowing one to measure the pressure while the temperature is constant. We also find “isobaric techniques” where the pressure is fixed, while we measure the temperature (e.g. the ebulliometer). In addition, we can find other synthetic techniques, such as the techniques involving the measurement of the densities. The full details and description concerning these different techniques can be found in (Dohrn et al. 2010; Fonseca et al. 2011) papers.
VLE equipment
Materials
Nine refrigerants were used to achieve VLE measurements, to study pure compounds vapour pressures, and binary mixtures phase behaviour. The refrigerants used for the VLE experiments, along with their ASHRAE number, chemical formula, CAS number, the name of the supplier and the purity of the product, are reported in Table 2.1. Table 2.1 Refrigerants used in VLE measurements a The purities given by the suppliers were verified by chromatographic analysis. For the R1233zd(E) we found a purity different to the one announced by the supplier (purity verified is estimated to be 93%). Compound ASHRAE Number Formula CAS Number Supplier Purity Carbon dioxide R744 CO2 124-38-9 Air liquide >99.995% 2,3,3,3-Tetrafluoropropene R1234yf C3H2F4 754-12-1 Honeywell >99.5% 1,1,1,2-Tetrafluoroethane R134a C2H2F4 811-97-2 Climalife >99% 1,1-Difluoroethane R152a C2H4F2 75-37-6 Dehon >99% Trans-1-chloro,3,3,3- trifluoropropene R1233zd(E) C3F3H2Cl 102687-65-0 Synquest >97%a 2-chloro-3,3,3 – trifluoropropene R1233xf C3F3H2Cl 2730-62-3 Synquest >99% 1,1,1,3,3- pentafluoropropane R245fa C3H3F5 460-73-1 Honeywell >99% Trifluoroethane R23 C2H3F3 420-46-2 Climalife > 99.5% n-Propane R290 C3H8 74-98-6 Messer > 99.995% Chapter 2 – Experimental equipments
Apparatus
The equipment used for the VLE measurements is based on a static-analytic method with liquid and vapour phase sampling using a capillary sampler ROLSITM. The equipment can be categorized under “Analytical technique, with sampling, isothermal AnT” according to (Dohrn et al. 2010) classification. The main part of the apparatus is the equilibrium cell, where the two-phase equilibrium takes place. The flow diagram of the apparatus is displayed in Figure 2.2 below. The apparatus is equipped with a thermo-regulated liquid bath where the equilibrium cell is immersed. The bath ensures the control of the temperature within 0.01 K. The temperature measurements inside the equilibrium cell is performed using two platinum resistance thermometer probes (Pt100) (Valtz et al. 2003), one to measure the temperature at the top of the cell, and the other for the temperature at the bottom of the cell. Two other EC ST GC TR SD V1 C1 LV1 EC PP MS TR PT LV2 TP PT TR V2 VP V3 LB SC PC LS VS V4 C2 V6 VP PP TP TR V5 Figure 2.2 Flow diagram of the static-analytic apparatus (Juntarachat et al. 2014). EC: equilibrium cell; LV: loading valve; MS: magnetic stirrer; PP: platinum resistance thermometer probe; PT: pressure transducer; RT: temperature regulator; LB: liquid bath; TP: thermal press; C1: more volatile compound; C2: less volatile compound; V: valve; GC: gas chromatograph; LS: liquid sampler; VS: vapor sampler; SC: sample controlling; PC: personal computer; VP: vacuum pump. Chapter 2 – Experimental equipments 25 temperature probes are used to control the temperature inside the thermal presses used to load the chemical products into the equilibrium cell. The Pt100 probes are connected to a data acquisition unit (HP34970A). The Pt100 probes are calibrated against a 25 Ω reference platinum resistance thermometer (Pt25 – Hart Scientific). The Pt25 reference probe was calibrated by the “Laboratoire National d’Essais de Paris” based on the 1990 International Temperature Scale (ITS 90). The temperature accuracy is estimated to be within ± 0.03 K. The pressure is measured using two pressure transducers, one for low pressures (DRUCK, 0 – 30 bar), and another for high pressures (DRUCK, 0 – 300 bar) connected to the same data acquisition unit (HP34970A). The pressure transducers are calibrated against a pressure automated calibrator (GE Sensing, model PACE 5000). The pressure accuracy is estimated to be within ± 0.004 bar. The analytical work is performed using a gas chromatograph (VARIAN, model CP-3800) equipped with a thermal conductivity detector (TCD). The analytical column used for most of the measurements is a RESTEK, 1% RT-1000 on Carboblack B, 60/80 mesh (length: 2.4m, diameter: 2mm from Restek, ID Silcosteel). The TCD is calibrated by introducing manually known amounts of each pure compound (for the system studied) through an automatic syringe in the injector of the gas chromatograph.
Sensors Calibrations
Temperature probes calibration
As mentioned before, the calibration of the Pt100 probes is performed using a 25 Ω reference platinum resistance thermometer (Pt25 – Hart Scientific). The corrected values (Tcal) are obtained from the polynomial relation (1st or 2nd order) relating the values read through the temperature probes (Tread) to the values obtained with the reference probe (Tref). Example of the calibration is given in Figure 2.3, representing the calibration of the temperature probe at the bottom of the cell, where we have the representation of Tref as a function of Tread and the representation of the deviation between Tref and Tcal as a function of Tref. Chapter 2 – Experimental equipments 26 Figure 2.3 Calibration of the temperature probe. (a): Second-order relation between the read and reference temperatures. (b): Deviations from the reference temperature, resulting from the use of a second-order relation.
Pressure transducers calibration
As mentioned before, the pressure transducers are calibrated against a pressure automated calibrator (GE Sensing, model PACE 5000). The corrected value (Pcal) are obtained from the polynomial relation (1st or 2nd order) relating the values read through the temperature probes (Pread) to the values obtained with the reference probe (Pref). Example of the calibration is given in Figure 2.4, representing the calibration of the lowpressure transducer (DRUCK, 0 – 30 bar), where we have the representation of Pref as a function of Pread, and the representation of the deviation between Pref and Pcal as a function of Pref.
1. Introduction |