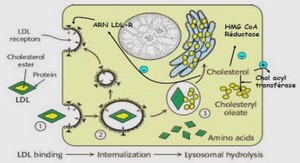
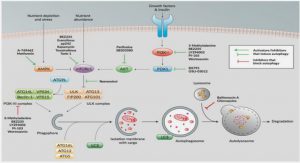
Mothers reduce egg provisioning with age
Reproductive investment is an essential feature in the study of life histories (Smith & Fretwell 1974; Charlesworth 1980; Perrin & Sibly 1993; Einum & Fleming 2000; Roff 2002) and is fundamental to numerous fields of research in behavioural, evolutionary and population ecology (Rivero & Casas 1999a; Hochberg & Ives 2000; LaMontagne & McCauley 2001; Roff 2002). Several optimality models have been developed to predict the optimal maternal investment per offspring on the basis that reproductive strategies evolve to maximize the number of viable offspring, and thus parental fitness (Lack 1947; Stearns 1992). One of the most widely used predictors of reproductive investment is egg size (Winkler & Wallin 1987; Sinervo & Licht 1991; Bernardo 1996). This approach assumes that 1) large eggs produce offspring with higher fitness and 2) large eggs are more costly to produce (Bernardo 1996; McIntyre & Gooding 2000; Roff 2002). These assumptions are however largely contingent on egg nutrient composition being correlated with egg size, an a priori that has rarely been tested (see e.g. Einum & Fleming 2000 for an exception). Egg size and composition are however not necessarily correlated and variation in egg composition can be ecologically and evolutionarily more important than variation in egg size (Begon & Parker 1986; Bernardo 1996; Fox & Czesak 2000). Furthermore, numerous studies have demonstrated that females could vary their investment per egg (e.g., as a function of age), a pattern reflecting physiological constraints on egg production or an adaptive strategy (Begon & Parker 1986; Clutton-Brock & Godfray 1993; Bernardo 1996; Roff 2002). In this study, we investigated the physiology of maternal reproductive investment in the parasitoid Eupelmus vuilletti (Hymenoptera, Eupelmidae). We aimed to (1) quantify the amount of resources allocated to eggs throughout the lifetime of the female using both egg size and biochemical composition as indicators of maternal investment, and (2) explore the relationship between maternal reproductive investment per egg and neonate larval fitness. Materials and methods Eupelmus vuilletti (CRW) (Hymenoptera, Eupelmidae) is a solitary ectoparasitoid that feeds and oviposits on the third and fourth larval instars of Callosobruchus maculatus Reduction of egg provisioning with age 42 (Coleoptera, Bruchidae). These Coleoptera develop during their post-embryonic instar within the pods and seeds of Vigna unguiculata (Fabaceae). All experiments were carried out on insects that had been raised in the laboratory at 23°C, a 13 L: 11 D photoperiod, and 75% humidity. Hosts for the experiment, were extracted from the seeds, and placed individually inside gelatin capsules (for details see Giron et al. 2002). This system does not alter the natural oviposition pattern of females nor their life expectancy (Giron et al. 2002) and allows for the number and the developmental stage of the hosts to be controlled. Furthermore, it facilitates the collection of eggs laid by the parasitoid. As a female lays several eggs per day, we will use the term ‘age’ for a female’s age and ‘oviposition rank’ (position in the laying sequence) for an egg’s status.
Egg size and composition
To determine egg composition during a female’s life span, we carried out a first experiment whereby 70 newly emerged females were placed individually inside a Petri-dish (diameter 5.5 cm) and given one host per hour for a total of six hours (between 9am and 3pm, which corresponds to peak daily reproductive activity; D. Giron personal observation). This was repeated until the death of the female. The gelatin capsules containing the hosts were collected each day. The length (L) and the width (W) of each egg was immediately measured with a micrometer, and the volume (V) of each egg was estimated by the equation V = (p x L x W²)/6 (Avelar 1993). Quantification of lipids and sugars in eggs was carried out using a modification of the colorimetric techniques developed by Van Handel (Van Handel 1993; Giron et al. 2002). Protein analysis was conducted using the Bradford assay procedure (Giron et al. 2002). The techniques used were not sensitive enough for individual analyses of eggs. Therefore, egg composition was determined using batches of five eggs of the same oviposition rank (position in the laying sequence) but from different, arbitrarily chosen, females. Each batch was used for the colorimetric analysis of only one type of nutrient (lipids, carbohydrates or proteins). This means that our results are correlational, and we cannot assess the actual covariation of these three nutrients within eggs. The analyses were carried out on eggs from rank 1 to 40 in the laying sequence, with between 1 and 3 batches per oviposition rank and per nutrient class (rank 1 to 25: 3 batches; rank 26 to 37: 2 batches, rank 38 to 40: 1 batch). Beyond rank 40, the number of eggs obtained was too low for analysis. We eva luated the mean energy content of the eggs in each rank by converting the mean nutrient content into Joules (conversion factors: 43 Chapitre 3 proteins 16.0 J/g; carbohydrates 16.0 J/g; lipids 37.5 J/g; see Rivero & Casas 1999a and McIntyre & Gooding 2000).
Correlation among egg size, egg composition and offspring fitness
To determine the effects of egg size on the survival of the resulting offspring, we carried out a second experiment whereby 30 females were allowed to oviposit in the same conditions as described above. The size of each egg laid by each female during its entire life was measured and then each egg was placed individually into a gelatin capsule (without a host) until eclosion. Once emerged, larval status (alive or dead) was checked on an hourly basis by detecting the presence of a heartbeat. For this purpose the heart was observed through the cuticule with the aid of a binocular microscope. The survival of neonate larvae in the absence of a food source has been previously used as an estimate of offspring fitness (see Guisande & Harris 1995 and Diss et al. 1996) and was chosen here for three reasons. First, many eggs are laid near, rather than on, the host (D. Giron, personal observation). Thus, the first hours before attachment has been secured and host fluids have been obtained are a crucial phase of a neonate’s life. Second, the survival criterion does not explicitly include differences in host quality, which can have a critical influence on offspring fitness (Godfray 1994). Finally, most of the studies that have failed to detect fitness advantages from large eggs have reared progeny in high-quality environments. Selection is however expected to be generally stronger in low-quality environments (Fox & Czesak 2000). The effect of egg size on offspring survival was analysed using generalized non-linear regression techniques (SYSTAT, Chicago USA). Egg composition was determined using batches of eggs. Therefore, correlation analyses between egg size and absolute egg composition were performed using the values obtained for each batch at each oviposition rank (n=120 batches). For estimating the relative composition as function of egg size, we based our correlation analyses on the mean values obtained at each oviposition rank (n=40 batches).
Results
Theoretical studies predict that reproductive investment per egg either stays constant, increases or decreases with age (Roff 2002). Hence, egg size, lipid, carbohydrate, protein and energy contents were expressed as percentages of the mean values of the first egg laid. Moreover, this makes visual comparison of the slopes in figures 1a and 2a-d easier. Females laid an average of 28.5±2.7 eggs over a period of 11.5±0.4 days. Egg size declined with position in the laying sequence and the last eggs laid were about 12% smaller than the first eggs (Figure 1a). Neonate larval survival, when deprived of food, was higher for larger eggs (Figure 1b). The sigmoidal shape of the relationship (non-linear regression, y = 26.32/1+74014.00*EXP(-1.37*x)) was assessed by checking for systematic linear or curvilinear behaviour of the residuals with respect to size. None was found and only 12 points of 760 had residual values higher than 1.96 (data not shown).Egg composition decreased during the life of a female (Figure 2a-2d). The pattern of decrease in nutrient content with rank was relatively similar to the pattern observed for egg size, with the exception of lipids, which reached a stable lower value after c.a. the 13th egg (approximately 0.24µg/egg) (Figure 2a) Lipid, carbohydrate and protein contents were correlated with egg size (lipid content r=0.67, n=120 batches and p<0.005; carbohydrate content r=0.35, n=120 batches and p<0.005; protein content r=0.75, n=120 batches and p<0.005). Moreover, the relative proportion of carbohydrate (mean+s.e. 26.05+0.46%; n=40) and protein (23.19+0.66%; n=40) varied significantly with egg size (carbohydrate percentage r=–0.49, n=40 and p<0.005; protein percentage r=0.64, n=40 and p<0.005). However, the lipid percentage (50.77+0.67%; n=40) did not vary significantly with egg size (lipid percentage r=–0.28, n=40 and p=0.080). These results suggest differences in the relative chemical compositions of small and large eggs.