Télécharger le fichier original (Mémoire de fin d’études)
Cadmium and zinc aqueous species in hydrothermal fluids
Major ligands for Cd and Zn in hydrothermal fluids
The major natural ligands capable of transporting Cd under hydrothermal conditions are OH-/H2O, Cl-, and HS-/H2S. The thermodynamic properties of cations Zn2+ and Cd2+ are very well known and recommended values of standard thermodynamic functions can be found in CODATA (1989). In aqueous solution the Zn2+ and Cd2+ cations undergo hydrolysis with formation of Zn(OH)n2-n and Cd(OH)n2-n hydroxide complexes, where 1 ≤ n ≤ 4. The stability constants of Zn and Cd hydroxide species at ambient T-P are well known from extensive experimental studies, and compilations of the thermodynamic properties of these complexes were reported (Baes and Mesmer, 1976; Wagman et al., 1982; Martell and Smith, 1998; Zhang and Muhammed, 2001). Based on these data and theoretical correlations with other metal hydroxide complexes, Shock et al. (1997) reported HKF parameters of Zn(OH)n2-n and Cd(OH)n2-n allowing calculations at elevated T-P (SUPCRT, http://geopig.asu.edu/index.html/). However, for zinc the predicted temperature dependence was shown to be too strong by recent experiments up to 300°C (Bénézeth et al., 2002), which indicate that these complexes may be much weakern at high T-P than predicted by Shock et al. (1997). It is thus expected that the currently available predictions for cadmium hydroxide complexes stabilities given by Shock et al. (1997) might suffer from the same flaw at elevated temperatures. To resolve the discrepancies about the role of hydroxide complexes in Cd and Zn transport by high-temperature fluids, additional experiments are required, particularly at temperatures above 300°C, which will be a part of this work.
A recent review of the available low-temperature experimental studies of Zn and Cd aqueous complexes with sulfide can be found in Rickard and Luther (2006). Despite the generally stronger chemical affinities of both metals for the “softer” sulfide ligand than for “borderline” chloride (e.g ., Pearson, 1963), sulfides complexes are unlikely to be important for these metals in acidic high-temperature hydrothermal solutions. The stabilities of Zn-S/HS complexes were shown to exhibit very weak temperature dependence, whereas the domain of predominance of Zn-Cl complexes expands rapidly with increasing temperature (Rickard and Luther, 2006; Tagirov et al., 2007). Despite the higher chemical affinity of Cd to the sulfide ligand compared to Zn, and the lack of experimental data for Cd-S complexes at temperatures above ambient, the Cd-S/HS complexes are also expected to be minor in comparison to CdCl species. This conclusion is dictated by the generally low sulfur concentrations in most natural hydrothermal fluids, typically less than 0.1 wt% (Barnes, 1979), whereas chloride is by far more abundant ligand in hydrothermal fluids, with concentrations attaining 10-30 wt% (Barnes, 1979). Moreover, in acidic and near-neutral solutions typical of Cd/Zn ore deposit formation, the concentration of charged HS- ligands is low compared to the neutral H2S0 which has a much weaker complexing capacity. Analyses of natural hydrothermal fluids (see above, Fig. I-4) indicate positive correlations between Zn and Cl concentrations, thus implying that chloride complexes are likely to be dominant for Zn and other base metals (e.g., Yardley, 2005). Thus, it can be assumed that chloride complexes play a main role in the both cadmium and zinc transport in hydrothermal settings. We discuss the available data on structures and stabilities of Cd-Cl and Zn-Cl complexes below.
Molecular structures of Cd and Zn complexes in aqueous solution
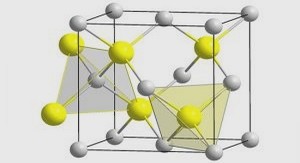
The structures of cadmium and zinc aqueous species in chloride solutions were studied using X-ray diffraction (XRD), Nuclear Magnetic Resonance (NMR), Raman, Infrared, and X-ray absorption spectroscopies (XAS) (e.g., Irish et al., 1963; Waters et al., 1973; Drakenberg et al., 1978; Fontana et al., 1978; Shuvell and Dunham, 1978; Ackerman et al., 1979; Caminiti et al., 1980; Buback, 1983; Paschina et al, 1983; Anderson et al., 1995; Mosselmans et al., 1996; Mayanovic et al., 1999; Bassett et al. 2000; Seward and Driesner, 2004; Liu et al. 2007) and computational theoretical methods (e.g., 1992; Butterworth et al., 1992; Rudolph and Pye, 1998). Most of these studies agree about a) octahedral structures of the ions Cd2+ and Zn2+ which are coordinated by 6 water molecules in the nearest atomic sphere of the metal; b) tetrahedral structures of CdCl42- and ZnCl42-; c) the presence of water molecules in the first coordination shell of intermediate chloride species CdClnH2Om2-n and ZnClnH2Om2-n; d) the decrease of the average number of water molecules with increasing T and Cl concentration in such species; and e) the coordination change from octahedral to tetrahedral with increasing Cl concentration in solution for both zinc and cadmium chloride complexes. However, these even rather accurate and detailed spectroscopic measurements at ambient conditions are still difficult to interpret in terms of formation of a mixture of Zn and Cd chloride species in solution, and to determine the exact ‘moment’ for the octahedral- to-tetrahedral transition as a function of ligation number. For example for zinc, this transition is likely to occur at the formation of the second chloride complex ZnCl20 (Buback, 1983; Mayanovic et al., 1999; Bassett et al., 2000; Liu et al., 2007). For cadmium the coordination change was proposed at the formation of the second CdCl20(aq) or third CdCl3- complex, depending on the solvent (Ahrland, 1979). It should be noted, that the formation of low-coordination tetrahedral species with both increasing mCl and T, demonstrated for other base metals (e.g., Fe, Zn, Co, Ni), is expected to significantly increase ore-metal solubility (e.g., Crerar et al., 1985; Susak and Crerar, 1985).
The structure of cadmium and zinc aqueous species at temperatures above ambient remain very poorly known. Few scarce structural data were reported for Cd-Cl complexes using XAS, but they explored very limited T and mCl ranges (mCl ≤ 1m, T ≤ 250°C, Mosselmans et al., 1996; Seward and Driesner, 2004). These data do not allow derivation of the identity and stability of the dominant Cd-Cl complexes. For zinc, more spectroscopic studies at elevated T are available. The major Zn-Cl complexes identified by Raman and X-ray absorption spectroscopy at T above 300°C are ZnCl2(H2O)20 and ZnCl42- (Buback, 1983; Mayanovic et al., 1999; Bassett et al., 2000; Liu et al., 2007). However, these data are debatable because they do not cover systematically the sufficient range of mCl and T to reveal unambiguously structures and stoichiometries of the different possible Zn chloride species. The major limitations of the spectroscopic methods are: a) the difficulty to detect the presence of oxygen atoms (i.e., water or hydroxide ligands) in the first coordination shell of the metal in its chloride complexes; b) the difficulty to interpret the XAS spectra in case of a mixture of species in solution; and c) the lack of confrontation of the structural spectroscopic data with thermodynamic information about the complex stabilities. The latter limitation is probably the most important one and a rigorous confrontation between structural and thermodynamic data has not been done so far for Zn and Cd, to the best of our knowledge. Unfortunately, the existing thermodynamic data for chloride complexes of both elements at elevated T-P appear to be surprisingly contradictory, prohibiting their comparison with the few structural results discussed above. In the two following sections, we will present the state of the art about the stabilities and stoichiometries of the major aqueous Cd and Zn chloride complexes reported in the literature.
Thermodynamic properties of cadmium chloride complexes
Numerous experimental studies were performed at T ≤ 50 – 100°C to determine the stability constants of stepwise formation for chloride complexes CdCln2-n, where n ranges between 1 and 4 (because the water activity is close to one in most studies of aqueous solutions, structural H2O molecules present in these complexes are omitted for clarity). These studies have been conducted using potentiometry (e.g., Vanderzee and Dawson, 1953; Reilly and Stokes, 1970; Gutz and Neves, 1985; Tomaš et al., 2001), calorimetry (e.q., Gerdi ng, 1966; Provost and Wulf, 1970), polarography (e.q., Eriksson, 1953), solubility (e.q., King, 1949), ebulliometry (e.q., Oliveira, 1979), and ultrasonic absorption measurements (Valleau and Turner, 1964). The majority of databases and review compilations show a decent agreement as for the stability constants values for the first three cadmium chloride complexes at 25°C and 1 bar (Table I-2, La timer, 1952; Glushko, 1972; Wagman et al., 1982; Sverjensky et al., 1997; Archer, 1998; Pivovarov, 2005). The only exceptions are a) the NBS data of Wagman et al. (1982) that are likely to contain errors as it was demonstrated by a more recent review of these data by Archer (1998), and b) an old report of the CdCl+ formation constant recommended by Latimer (1952). Data on the CdCl42- stability constant, however, are scarcer than those for the lower Cl number species. Nevertheless, the two existing reviews of Sverjensky et al. (1997) and Pivovarov (2005) report comparable values (within < 0.3 log unit, Table I-2). The distribution of cadmium species in a H2O-NaCl-HCl solution at pH ~2 as a function of total mCl at 25°C and 1 bar calculated according to Sverjensky et al. (1997) is presented in Fig.I-6a.
In contrast, the available data from different experimental and theoretical studies at temperatures above ambient are surprisingly inconsistent and display large variations in the temperature dependence of the stability constants for all Cd-Cl species. This can be seen in Table I-3, where we compared the reported values of the standard partial entropy S0298.15 for each chloride complex, derived from the log Ki vs T dependence between 20 and 100°C in different studi es. It can be seen in this Table that differences in the S0298.15 values for a same complex attain almost 40 cal × mol-1×K-1 (e.g., for CdCl3- between Latimer, 1952 and Sverjensky et al., 1997). Such a difference in S0298.15 corresponds to a difference of more than 2.5 orders of magnitude of the CdCl3- stability constant when extrapolating these data to 150°C. The situation is even worse at higher temperatures, typical of Zn and Cd ore formation (~200-400°C). For example, onl y one experimental work at T up to 250°C is available for the first chloride complex CdCl+ (Palmer et al., 2000) , to the best of our knowledge.
This brief analysis of the available data shows that although the stability constants of all cadmium chloride complexes at ambient conditions are accurately constrained by numerous experimental measurements, large disagreements exist as to the temperature dependence of the formation constants. The discrepant entropy (and enthalpy) values for most Cd-Cl complexes do not allow reliable extrapolations above 100-150°C. The SUPCRT thermodynamic database (http://geopig.asu.edu/index.html/) widely used by geologists in modeling hydrothermal fluids contains a complete set of the HKF equation-of-state parameters for the four Cd-Cl species derived from the ambient T-P data and using empirical correlations amongst thermodynamic values of different metal complexes (Sverjensky et al., 1997). However, as we showed above, these data are in clear disagreement with other sources (Table I-3) and thus cannot be used with confidence at elevated T-P. Thus, there is an urgent need for systematic experimental data of the identities and stabilities of Cd-Cl species in high-temperature solutions.
Thermodynamic properties of zinc chloride complexes
Zinc complexing with chloride in aqueous solution in a wide T range has attracted far more considerable attention than that of his “little bro ther” cadmium. As a result, numerous experimental and modeling studies are available on the stability of zinc chloride complexes ZnCln2-n, where n ranges from 1 to 4. The available formation constants for these zinc chloride complexes at 25°C and 1 bar are given in Table I-4. Despite the large volume of available experimental and theoretical studies listed in this Table, the stabilities of all Zn-Cl complexes exhibit surprisingly larger variations amongst different data sources than their Cd analogues (Table I-2), attaining more than one log unit (in terms of log10n). This is due to the fact that Zn-Cl complex stabilities are 1-2 orders of magnitude lower that their Cd counterparts (e.g., Fig. I-6). This renders it more difficult accurate direct measurement of Zn-Cl interactions at ambient conditions, so that most stability constants reported in Table I-4 are based on extrapolations from high-temperature conditions where the stability of Zn-Cl species is higher. For ZnCl+ and ZnCl20(aq) most reported data are in decent agreement, within 0.2-0.4 log units, except the old NBS report (Wagman et al., 1982) and the revision of Pivovarov (2005) who used few selected sources of data. Their values of stability constants derived by the original authors (Ruaya and Seward, 1986; Bourcier and Barnes, 1987; Plyasunov and Ivanov, 1991) from three high-temperature solubility studies are in excellent agreement within 0.1 log unit. The data for Zn-Cl species from the most popular amongst geologists database SUPCRT (http://geopig.asu.edu/index.html/), based on a critical revision by Sverjensky et al. (1997) of selected solubility studies (e.g., Ruaya and Seward, 1986) are slightly different from the original values cited above (by ~ 0.2-0.4 log units) likely because of different extrapolation procedures used. For the higher ligand number complexes, ZnCl3- and ZnCl42-, the agreement is worse, and differences between the SUPCRT database and the solubility studies cited above attain more than one order of magnitude in terms of overall stability constant (β0n). Note also that these discrepancies further increase for the ‘last’ ZnCl 42- species, for which the limited amount of available data does not allow derivation of the consistent value.
In contrast with Cd, the stability of Zn-Cl complexes at hydrothermal temperatures (typically to 350°C) has been a subject of several solubility studies summarized in Table I-5. The comparison of these data shows that while the data for the uncharged ZnCl20(aq) complex are very consistent over a wide T range (with a single exception of Plyasuvov and Ivanov, 1991), the situation for the other complexes is less good, with differences attaining 4 orders of magnitude (!) at T > 300°C for ZnCl+ and ZnCl42-. The distribution of Zn-Cl complexes in a 0.1m and 2m NaCl solutions at 350°C/P sat according to different original solubility studies are illustrated in Fig. I-7. The values suggested by Ruaya and Seward (1986), Bourcier and Barnes (1987) and Wesolowski et al. (1998) imply that ZnCl+ and ZnCl20(aq) are the dominant species at chloride concentrations typical of most Zn-bearing ore-forming fluids (0.1-2.0m NaCl). This conclusion is also supported by the solubility data of Cygan et al. (1994) at supercritical temperatures (300-600°C). In contrast, the stability cons tant values of Plyasunov and Ivanov (1991) suggest that ZnCl42- is by far the major species at mCl > 1m. It should be emphasized that the identity (~ number of Cl ligands) of the dominant Zn species exerts a major control of Zn-bearing mineral solubilities as a function of pH and chloride concentration. The discrepancies apparent in Fig. I-7 imply that in most saline hydrothermal fluids, the solubility dependence of Zn sulfides and silicates as a function of acidity and chlorinity will be different if modeled using different data sources (e.g., Ruaya and Seward, 1986 versus Plyasunov and Ivanov, 1991).
Table des matières
Chapter I. INTRODUCTION
I-1. Geochemistry of cadmium and zinc
I-2. Cadmium and zinc aqueous species in hydrothermal fluids
I-3. Aims of the present study
I-4. Manuscript organization
Chapter II. METHODS
II-1. Potentiometry
II-2. Solubility
II-2-1. Hydrothermal pretreatment of CdO and ZnO solids
II-2-2. CdO and ZnO solubility in pure water and low-saline solutions at 350-400°C and 220-400 bar
II-2-3. CdO and CdS solubility in saline solutions at 400°C and 600 bar
II-3. Vapor-liquid partitioning
II-4. X-ray absorption fine structure spectroscopy
II-5. Sampling and analyses of natural high–temperature fumaroles gases (Kudryavy volcano, Kuril Islands, Russia)
II-6. Analytical methods
II-7. Thermodynamic calculations
Chapter III. POTENTIOMETRIC STUDY OF CADMIUM CHLORIDE COMLEXES FROM 1 TO 1000 BAR AT 25°C
Bazarkina, E.F., Zotov, A.V., Akinfiev, N.N., »Potentiomentric study of cadmium chloride complexes from 1 to 1000 bar at 25°C » submitted to Geology of Ore deposits in August 2009
Extended abstract (in English)
Full text of article (in Russian)
Chapter IV. STRUCTURE AND STABILITY OF CADMIUM CHLORIDE COMPLEXES IN HYDROTHERMAL FLUIDS
Bazarkina E.F., Pokrovski G.S., Zotov A.V., Hazemann, J.-L., « Structure and stability of cadmium chloride complexes in hydrothermal fluids » submitted to Chemical Geology in October 2009
Full text of article (in English)
Suplemmentary Electronic Information (in English)
Chapter V. MODELING OF Cd/Zn RATIOS IN HYDROTHERMAL FLUIDS AND VAPORS AND COMPARISON WITH NATURAL OBSERVATIONS
V-1. Derivation of thermodynamic properties for Cd and Zn chloride complexes and modeling of Cd/Zn ratios in dense saline hydrothermal fluids
V-2. Solubility of CdO and ZnO at 350-400°C and 220-400 bar
V-3. Distribution of Cd and Zn between the coexisting vapor and liquid phases in the water-salt system
V-4. Cd and Zn in volcanic gases of Kudryavy volcano: brief results of a field work in September 2006
Supplementary Tables for Chapter V
Chapter VI. CONCLUSIONS AND PERSPECTIVES
References