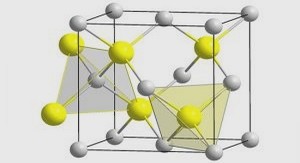
Optimisation d’hétérostructures à multipuits quantiques InGaN sur sous-couche InGaN pour diodes électroluminescentes
EPITAXIAL GROWTH AND CHARACTERIZATION TECHNIQUES
Epitaxial Growth: Metal Organic Vapour Phase Epitaxy (MOVPE) MOVPE is a crystal-growth technology that allows the growth of layers of high quality semiconductor crystal with very precise thickness, composition control and reproducibility, in the nanometer range, even near atomic-layer precision. MOVPE is also known as MOCVD (Metal Organic Chemical Vapour Deposition) or OMVPE (Organo Metallic Vapour Phase Epitaxy) or OMCVD (Organo Metallic Chemical Vapour Deposition). In MOVPE technique, the liquid or solid metalorganic compounds which have fairly low vapour pressure can be used as gaseous precursor along with group-V hydrides by using bubbler and carrier gas technology. The upscalability, excellent control, and relatively high growth rate (higher than MBE) have made MOVPE the first choice for III-nitride epitaxy in electronic and optoelectronic industries. The organometallic compounds of group-III metals and hydrides of groupV elements are transported in vapour phase into a reaction chamber where they react. The global reaction can be generalized as, 𝑅3𝑀(𝑔𝑎𝑠 ) + 𝐸𝐻3 𝑔𝑎𝑠 → 𝑀𝐸(𝑠𝑜𝑙𝑖𝑑 ) + 3𝑅𝐻4 𝑔𝑎𝑠 Where M and E symbolize the group-III and group-V elements respectively, R is the type of organic radical (typically an alkyl) the group-III element is attached to, and H is elementary hydrogen. For example, GaN is typically formed by the reaction between trimethylgallium (TMGa) or (CH3)3Ga and ammonia (NH3 ), (𝐶𝐻3)3𝐺𝑎(𝑔𝑎𝑠 ) + 𝑁𝐻3(𝑔𝑎𝑠 ) → 𝐺𝑎𝑁(𝑠𝑜𝑙𝑖𝑑 ) + 3𝐶𝐻4 𝑔𝑎𝑠 66 And the formation of InxGa1-xN requires the additional precursor trimethylindium (TMIn) or (CH3)3 In, together with TMGa and NH3. Typically, metalorganic (MO) precursors’ temperatures of use range from −10 oC to 50 oC. Each precursor is stored in a sealed metal container called “bubbler”. Within the bubbler, the precursor, either liquid or solid, remains at equilibrium with its vapour. Bubbler temperature is controlled by placing the bubbler in a temperaturecontrolled water or glycol bath. This temperature controls the liquid-vapour or solidvapour equilibrium, hence the partial vapour pressure of the precursor in the bubbler. Elements like Oxygen (O), Sulphur (S) etc. would lead to a contamination in the epitaxial layers and Chlorine (Cl) could possibly change the growth regime and favour corrosion of the reactor metallic pieces and should therefore be avoided in the MO molecule [46]. The vapour pressures of some MOs used in this thesis work are shown in Figure 21. The precursor molar flow rate Φm can be expressed in relation with the precursor partial pressure Pmo,partial, the total pressure Ptot, the bubbler temperature T, and the total volumetric flow of gas in the bubbler Φv,tot, 𝑚 = 1 𝑅𝑇 𝑃𝑚𝑜 ,𝑝𝑎𝑟𝑡𝑖𝑎𝑙 𝑃𝑡𝑜𝑡 𝑣,𝑡𝑜𝑡 Where, R is the molar gas constant for ideal-gas. The units of molar and volumetric flow rates are molmin-1 and sccm (standard cubic centimeter per minute). Finally, the partial pressure of the precursor depends on the bubbler temperature according to following equation, 67 Figure 21: Temperature dependent vapour pressure data of some metalorganic precursors used in this thesis work (after [17]). 𝑃𝑚𝑜 ,𝑝𝑎𝑟𝑡𝑖𝑎𝑙 = 10𝑎− 𝑏 𝑇 The values of the parameters a and b for the organometallic precursors used in this thesis work are given in Table 5. Table 5: Parameters for computation of the partial pressures of metalorganic precursors [49] Precursor a b (K) Boiling temperature ( oC) Melting temperature ( oC) TMGa 8.07 1703 56 -15 TMIn 10.52 3014 134 88 68 Hydride precursors of group-V elements are typically gaseous at room temperature and atmospheric pressure. The amount of those elements is controlled by controlling the precursor gas flow injected into the main stream of carrier gas H2 and / or N2. From the calculated debits of precursors flow rates, the V/III ratio and the III/(III+V) ratio can be calculated. The basic components of an MOVPE system are (Figure 22) [46]: Channels connected with metalorganic sources that provide the controlled flow of a carrier gas carrying an intended amount of precursor material. The carrier gas can be switched from the bubbler to the by-pass channel in order to keep the carrier gas flowing in a stand-by mode. Figure 22: General schematic of an MOVPE system [46]. Channels and sources for the controlled flow of required group-V hydrides. For each source, automatic (2/3/5-way) valves, allowing access either to the reactor chamber or to the vent line. The switching is optimized so that the gas 69 flow coming from a precursor source is stable all along the period of use of the precursor. Reactor chamber (horizontal or vertical) usually made of quartz or surrounded by quartz or stainless steel, where the epitaxial growth takes place. This chamber is fed by precursor gases and also by inert carrier gases. Programmable logic control (PLC) unit with software that controls the gas and precursor flows and injection timing, the temperature and the pressure. The flow of the carrier gas is controlled by electronic mass flow controllers (MFCs). The carrier gas can be switched from the bubbler to the by-pass channel in order to keep the carrier gas flowing in a stand-by mode [46]. Vacuum system (pump, pressure controller, valves etc.) to control and stabilize the pressure during growth. Exhaust system where the toxic exhaust gases are purified to remove any toxic fractions before releasing it to the atmosphere. A susceptor in the reactor chamber (usually made of graphite with SiC coating) where the substrate is placed and the epitaxial growth takes place on the substrate. The growth rate explicitly depends on the reactor temperature and can be related to three temperature regimes [46]. In the low temperature regime, the growth rate is limited by the chemical reaction (decomposition or desorption on the surface) rate which depends on the temperature according to the Arrhenius law: 𝑅 = 𝐴𝑒 −𝐸𝑎 𝑘𝑇 , where A is the pre-exponential factor, R represents the chemical reaction rate, Ea is the activation energy, k is Boltzmann constant and T is the temperature in K. In the medium temperature range (800 oC to 1200 oC for GaN epitaxy from TMGa and 70 NH3), the growth rate is dependent on precursor supply and almost independent of temperature. Growth in MOVPE reactors takes place in this so called “mass transport limited” regime [46]. For the epitaxy of most III-V compounds, including GaN, since group-V elements are much more volatile than group-III ones, the growth rate is mostly controlled by the partial pressure of group-III elements in the reaction chamber. In the high temperature regime (>1200 oC), thermodynamic factors, such as desorption and dissociation of the crystals, tend to limit the growth rate with increasing temperature. In order to grow the epi-layers stoichiometrically, it is necessary to control the ratio of the precursors, e.g. V/III ratio. Figure 23: Growth rate (log scale) as a function of inverse temperature (schematically).
Basic growth principle
The epitaxial growth in MOVPE is governed by diffusion processes that rely on the vapour transport of precursors in a heated zone, and it is conducted under near thermodynamic equilibrium conditions [44]. After the carrier gas being mixed with vapours of the precursors and being transported close to the heated substrate surface, surface chemical reactions take place. The schematic of different reactions during IIInitride epitaxial growth process is illustrated in Figure 24. 71 Figure 24: Illustration of epitaxial growth by MOVPE [44]. The heated organic precursor molecules then decompose in the absence of O2 or any halogen (pyrolysis) [44]. The resulting species diffuse through the boundary layer to the growth surface and attach onto the substrate by physisorption [44]. The species can desorb, migrate, or react with other surface species at this moment. To form a new layer, the species can diffuse on the surface and bound tightly at the bottom of a growth step, or nucleate at other positions to form islands [44]. The by-products from the chemical reactions are flowed out of the reaction zone to the exhaust by the carrier gas. There are also parasitic reactions that take place between precursors in the gas phase that can reduce the incorporation efficiency. Moreover, the particles of the byproducts may fall on the substrate surface and thus impede the formation of single crystal and therefore degrade the crystal quality. MOVPE growth includes mass transport, hydrodynamics, thermodynamics and kinetics. Typically, the growth rate and the quality of the III-nitride epitaxial layers depend mainly on four parameters: temperature, pressure, V/III ratio (the ratio of NH3 flow rate to the total flow rate of III and V precursors) and total group-III flow. Moreover, the choice of carrier gas (H2 or N2) has also an impact on the growth result. 72 Growth temperature should be optimized. Sufficient growth temperature is required to give enough energy for the pyrolysis of precursors, the diffusion of the atoms on the substrate surface and activation of the reactions which controls the growth rate and surface structural properties. However, too high temperature can initiate desorption and decomposition. In addition, growth temperature also affects the amount of indium incorporation in III-nitride alloys, such as, in our case, in InGaN. To reduce the parasitic reactions, the growth pressure is kept low (15-400 mbar) [76]. For low V/III ratio, background doping would be increased possibly due to nitrogen vacancy which acts as an acceptor [29,77,78]. On the other hand, for high V/III ratio, parasitic reactions would be aggravated and surface mobility of adsorbed atoms would be hindered. For GaN growth, H2 is used as carrier gas because it has good thermal conductivity, enables faster diffusivity of precursor species and has better carbon-radical furbishing properties compared to those of N2. However, for InGaN growth, N2 is used since the presence of H2 enhances indium desorption. Moreover, in H2 environment, Mg-H complexes are formed during the growth of Mg doped p-type GaN.
Experimental Setup
T-shape MOVPE reactor
The samples for this thesis work were grown in a T-shape MOVPE reactor [76]. The design of this reactor is a combination of vertical loading and horizontal quartz tube: allowing the advantage of laminar flow along with loading distribution vertically. The schematic of the T-shape reactor chamber is shown in Figure 25. Figure 25: Schematic structure (cross-section) of the T-shape MOVPE reactor chamber. An RF induction-heated graphite susceptor is used to heat samples up to the target temperature (for example, 1040 oC for typical GaN growth). Several numbers of thermocouples are set around the reactor chamber to measure temperatures of different zones. A high vacuum and pumping system controls the pressure inside the reactor between 60 torr to 750 torr. Photographs of the actual reactor and reaction chamber are given in Figure 26. Figure 26: Photographs of the reactor quartz tube (a) and the complete T-shape MOVPE reactor with gas panels (b). 74 An Epieye reflectometry setup was used to in-situ monitor epi-layer thickness during the growth. The principle is to measure the intensity of a laser beam that is reflected off the sample. Figure 27: Typical in-situ reflectometry for GaN growth by MOVPE. This intensity oscillates over time as a result of interferences because of the difference of the refractive index between the nitride films and the sapphire substrate (Figure 27). The laser of 633 nm wavelength is placed vertically on top of the metal cover of the reactor. The growth rate can be calculated by the following equation, 𝑔𝑟𝑜𝑤𝑡 𝑟𝑎𝑡𝑒 = 2𝑛𝑡 Where, λ is the wavelength of the laser beam, n is refractive index of the epi-layer and t is the time interval between two maxima or two minima of oscillations. The intensity of the laser reflection is the measure of the film quality as well, governed by the equation [44], 𝑅 = 𝑅𝑜 𝑒 − 4𝜋 75 Where R is the mean value of reflectance oscillations, λ is the used wavelength and σ refers to the root mean square of roughness. A reduction of the ratio between maxima and minima of the reflection intensity indicates the increase of surface roughness. Detail of the T-shape reactor can be found in [76]. 2.2.2 Aixtron Close Coupled Showerhead (CCS) 3 2” reactor The Aixtron CCS reactor was used basically to grow the GaN templates on sapphire substrates. Moreover, growth optimization of some of the MQW structures was also carried out in the CCS. The sketch and actual photograph of the CCS reactor in our lab are shown in Figure 28.
RESUME |