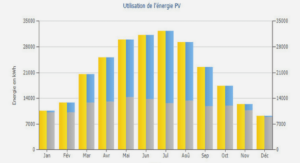
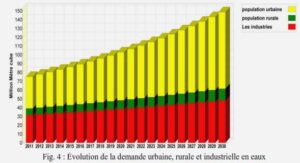
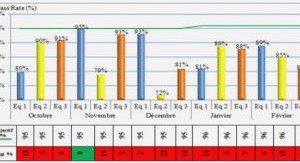
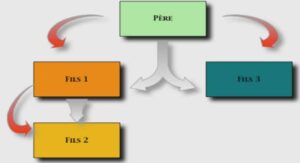
Structures et variations des réseaux trophiques des
poissons en zones côtières
Origin of and variability in organic matter in the Canche estuary
As estuaries are complex and changing ecosystems, it is challenging to distinguish the sources of organic matterat the base of food webs (Pasquaud et al., 2008; Selleslagh and Amara, 2015). Organic matter is a major component of suspended particles and fine sediment particles that determine many biogeochemical processes in marine environments (Bernasconi et al., 1997; Chen et al., 2012). Two natural sources of organic matter are generally considered in coastal ecosystems: allochthonous inputs and autochthonous production (Antonio and Richoux, 2014; Luo et al., 2016). Major sources of autochthonous organic matter include phytoplankton and aquatic macrophytes (Dalu and Froneman, 2016; Pearson et al., 2015). In estuarine ecosystems, organic matter of terrestrial origin is a major contributor to allochthonous organic matter (Duan et al., 2014; Lu et al., 2013). Analysis of the carbon and nitrogen stable isotopic compositions of estuarine organic matter can identify their contributions to the food web (Darnaude et al., 2004; Evans et al., 2019). Generally, POM in estuaries is composed of river POM (mixture of terrestrial POM and freshwater phytoplankton), estuarine-produced and marine phytoplankton, resuspended microphytobenthos and diverse detritus (e.g. faeces, macrophytes), which can make interpretation difficult (Kang et al., 2006). In the Canche estuary, the POM stable isotopic signatures indicated a mixed organic matter composition that included freshwater/estuarine phytoplankton (δ 13C ranging from -29‰ to -20‰), with a clear increase in POM δ 13C values from fresh to marine waters, similar to previous estuarine studies in the Bay of Marennes-Oléron (Riera and Richard, 1996), the Vilaine estuary (Kostecki et al., 2010) and the Gironde estuary (Selleslagh et al., 2015). In the Canche estuary, salinity exhibits short-term changes, with a large amplitude from 0-35 due to the small size of the estuary, tide conditions, season and weather conditions (Amara et al., 2009; Selleslagh and Amara, 2008). MPB that live in intertidal flats in estuaries can contribute much of the total primary production in estuaries (Underwood and Kromkamp, 1999). MPB on intertidal flats is composed mainly of benthic diatoms (Méléder et al., 2007) and several studies have emphasised its key role in sustaining intertidal food webs (Christianen et al., 2017; Herman et al., 2000; Thrush et al., 2012). In the Canche estuary, the MPB is one of the main primary producers and could therefore be an important source of organic matter for benthic invertebrates. As in other European estuaries, the Canche MPB had the highest enriched carbon ratios (-17‰ to -15‰) among food sources, which allowed it to 31 be traced in its consumers (Moens et al., 2002; Moncreiff and Sullivan, 2001; Riera and Richard, 1996). SOM is a mixture of benthic and deposited pelagic microalgae, bacteria, aquatic and terrestrial plant debris and meiofauna. In the Canche estuary, SOM and POM δ 13C followed the same trend, with the lowest values upstream and highest values downstream due to the presence of freshwater phytoplankton, as measured in other nearby estuaries (Lambert et al., 2017; Middelburg and Nieuwenhuize, 1998). In estuaries, variations in amounts and origins of nutrients such as nitrogen are common along salinity gradients, with a decrease in concentrations from fresh to marine waters due to mixing, which can be traced in food webs (Baeta et al., 2009; Connolly et al., 2013). The extent of nutrient mixing in estuaries varies spatially according to estuary size, and temporally at the seasonal and daily scales due to changing tides, wind, precipitation and temperature (Baeta et al., 2009; Hoeinghaus et al., 2011; Lautenschlager et al., 2014). δ 15N can be an accurate tracer for nitrogen inputs that originate from untreated domestic, industrial and/or agricultural activities that are incorporated in the food web through assimilation by primary producers (Fry, 2002). In the Canche estuary, POM, SOM and MPB δ 15N were similar along the salinity gradient during the same season, highlighting the relatively low nitrogen input from the watershed (Guelinckx et al., 2006) due to the short length of the Canche river and the low human modification of its catchment (Amara et al., 2007; Durou et al., 2007).
Structure of invertebrate communities
Two benthic communities were found in the Canche estuary: i) S. squamata/E.pulchra/Bathyporeia spp. (EUNIS A2.223), which corresponds to estuarine midshore medium-fine sand, and ii) H. diversicolor/S. plana on the upper shore mud banks (EUNIS A2.24; Rolet et al., 2015). Outside the estuary, Sainte Cécile and Le Touquet beaches were characterized by a low-shore fine sand N. cirrosa/S. squamata community (EUNIS A2.23), while the subtidal site had a muddy fine sand Abra alba/D. vittatus/F. fabula community (Desroy et al., 2003; Rolet et al., 2015). This distribution of benthic invertebrate communities is also present in the nearby estuaries of the Authie and Somme Rivers (Rolet et al., 2015). Biomasses inside the estuary and on adjacent beaches were lower (2.6-6.0 g AFDW.m-2 ) than in the subtidal A. alba community (150 g AFDW.m-2 ) dominated by the bivalve D. vittatus. In the EEC, benthic biomass within the A. alba community is heterogeneous, with a mean of 8.1 g AFDW.m-2 (Desroy et al., 2003), and a higher biomass ranging from 23.5-27.5 g AFDW.m-2 in the Seine Bay (Thiébaut et al., 1997) and from 45- 32 3 000 g AFDW.m-2 in Gravelines (Desroy et al., 2003; Dewarumez et al., 1992; Ghertsos et al., 2000). This high subtidal biomass of potential flatfish prey (mainly bivalves) outside the estuary created a major feeding ground for flatfish species. One objective of the study was to assess the origin of the food source consumed by potential prey throughout the estuary, from the intertidal zone upstream of the Canche estuary, with low benthic species diversity and biomass, to the subtidal zone outside the estuary, with higher species diversity and biomass. In the Canche estuary, suspension and deposit feeders dominated the benthic invertebrate biomass. Their feeding activity is an important connection between suspended and sedimented organic matter originating from POM, SOM or MPB (Little, 2000; Mann and Wetzel, 2000). However, it is often difficult to distinguish food sources of macrozoobenthos in estuaries due to spatio-temporal variability in the isotopic compositions of food sources along the salinity gradient, and because macrofauna feed on different food sources and have plastic feeding behaviour depending on the environmental conditions (Daggers et al., 2020; Herman et al., 2000). Nevertheless, benthic primary consumers had higher d13C (around – 20 to -16‰) than fresh water POM (around -30‰), revealing the latter’s low contribution to the trophic functioning of the estuary. This finding, even in the upstream of the estuary, may be dueto the relatively weak flow of the Canche River and consequently the small amount of organic matter that it carries (Selleslagh and Amara, 2008). Thus, we can hypothesize thatthe marine POM, SOM and MPB which d13C composition are around –22 to -15‰are the main food sources for the benthic community inside the estuary. However, it may be difficult to distinguish suspension and deposit feeders isotopically as their feeding behaviour does not provide information about the origin of their food; for example, suspension feeders can consume resuspended MPB, while deposit feeders can consume sediment POM (Kang et al., 2015).
Fish structure and seasonal variations
Two fish assemblages were observed, one inside the Canche estuary (P. minutus, P. microps and D. Labrax juveniles) and the other outside the estuary (B. luteum, S. solea and L. limanda juveniles). P. flesus and P. platessa juveniles occurred in both assemblages (Selleslagh and Amara, 2008). In both seasons in upstream Canche, P. flesus juveniles had the lowest δ 13C values, 5 to 8% away from that of the freshwater POM, revealing a slight contribution of organic matter from freshwater. This could be due to the diet of P. flesus juveniles, which is composed of meiofauna (nematodes, harpacticoides and ostracods (Aarnio et al., 1996; 33 Selleslagh and Amara, 2015)) that may consume SOM. Conversely, P. microps had the highest 13C values, which were similar to those of MPB, revealing the contribution of MPB to the feeding of P. microps prey. Previous studies indicated that P. microps feeds mainly on amphipods, polychaetes and meiofauna (Leclerc et al., 2014; Selleslagh and Amara, 2015). MPB production is highon the intertidal mud banks of the Canche estuary and provides food for the potential prey of P. microps. Inside the estuary, the other fish species (e.g. D. labrax, P. minutus or S. sprattus) had δ 13C values from -19 to -17‰ upstream to – 16‰ downstream, close to those of marine POM (around -20‰) and MPB (-16‰) revealing a food source originating from both marine POM and MPB. The seasonal comparison of fish SEA in the Canche estuary is informative, as the isotopic space occupied is smaller in spring than in fall. Thus, a wider range of prey appears to be consumed in the fall. This could be due to the higher biomass and diversity of coastal benthic invertebrates at the end of summer compared to the lower benthic biomass in spring (Rauch and Denis, 2008). In the fall, SEA of the two Pomatoschistus sp. did not overlap, unlike Platichthysflesus and Pleuronectes platessa that inhabit the Canche estuary, which confirms that their diet may differ (Salgado et al., 2004; Selleslagh and Amara, 2015). Conversely, the SEA of P. flesus and P. platessa overlapped in spring, perhaps due to the smaller amount of available prey (Pape and Bonhommeau, 2015). Outside the estuary, the δ 13C values of flatfish juveniles (B. luteum, S. solea, L. limanda) were ca. -16‰, revealing MPB and POM to be a major basic food source, except for P. platessa, which had much lower δ 13C values, similar to those found in the estuary. This suggests that P. platessa individuals caught outside the Canche estuary did not feed exclusively in the habitat in which they were collected, which indicates that this species has high mobility and habitat connectivity. Flatfish SEA indicates a slight isotopic niche overlap of S. solea, B. luteum and L. limanda, which suggests trophic segregation of the three species. Conversely, the SEA of P. flesus completely overlapped those of juveniles of these flatfish species, which indicates that P. flesus consumes a wider range of prey and may have trophic competition with the three other flatfish species. Juvenile fish in estuaries usually follow an opportunistic feeding strategy, which is driven by intra- and inter-specific competition (Brown et al., 2019; Post et al., 1999) and prey availability.
Conclusion
We showed a significant difference in invertebrate biomass between subtidal and intertidal sites, which influences the quality of the feeding ground for juvenile fish. This is a classic situation in European estuaries (Dubois et al., 2014). This result provides a new vision of the Canche estuary, which has been considered an important feeding ground for marine fish. Our study revealed that these continental inputs have a minor role in the functioning of the Canche estuary and that fish species might visit the estuary for reasons other than feeding, such as to avoid predation or because they are carried by the tide. We highlighted the need to take into account the whole small macrotidal estuary and adjacent ecosystems to better describe the flatfish nursery. This work demonstrated that potential prey and feeding sources for fish had habitat-specific compositions, which confirms the suitability of SIA for tracing fish movements, fidelity and connectivity inside and outside the Canche estuary for sites less than 10 km apart. Estuarine nursery feeding grounds, even in small estuaries, appear to be complex due to the mosaic of benthic communities (potential prey), which are related to the habitat (e.g. sediment type, foreshore position, salinity fluctuations) and to trophic competition and predation. Acknowledgements The authors would like to acknowledge the University of Littoral Opal Coast, the University of Badji Mokhtar Annaba and the Parc Naturel Marin des Estuaires Picards et de la Mer d’Opale that funded this work.
CHAPITRE 1 INTRODUCTION |