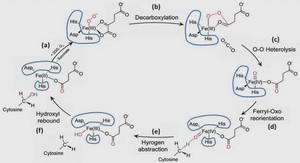
Étude de photocatalyseurs en couches minces de TiO2 pour la production d’H2
State of the Art, problematics and objectives
The energetic demand and climate change
Energy crises in the latter part of the 20th century, as well as the current increase in the cost of oil, emphasize the need for alternative sources of energy. The supply of secure, clean, sustainable energy is arguably the most important scientific and technical challenge facing humanity in the 21st century. 1,2 In 2001, worldwide primary energy consumption was 425 × 1018 J, which is equivalent to a rate of 13.5 TW. Yet, the global demand for energy is expected to continue to escalate in the coming decades. The Intergovernmental Panel on Climate Change, an organization established by the World Meteorological Organization and the United Nations Environment Program outlines an energetic global scenario depicted in Table 1.1. As predicted, the global population is expected to increase to 9.8 billion by 2050. With no changes in the average energy intensity, the world energy consumption rate would grow as well by 50% in 2050 to 27.6 TW. 4 However, the global average energy intensity has declined over the past 100 years, due to improvements in technology throughout the energy production, distribution, and end-use chain. 28 This 32 scenario raises a question about the economy security and the availability of energy sources on the planet. Table 1.1 World energy statistics and projections. Definition Units 2001 [1] 2050 [2] 2100 [2] Population Billion persons 6.145 9.8 10.4 Energy consumption rate TW 13.50 27.6 43.0 Carbon emission rate GtC year-1 6.57 11.0 13.3 Equivalent CO2 emission rate GtCO2 year-1 24.07 40.3 48.8 [1] Adapted from Ref. 29 [2] Scenario B2 adapted from Ref. 3 The global energy mix demand, defined by the primary energy consumption, has evolved over the last few centuries. After the industrial revolution, the rise of coal increased, followed by oil and gas, and by the turn of the 20th century, hydropower. Figure 1.1 shows the global energy consumption by source after 1965, note that the use of fossil fuels, which are the sum of coal, oil and gas, dominate the energetic consumption. In 2019, 84.3% of the global demand, was coming from fossil fuels, whereas the 15.7% left came from low-carbon sources, i.e., nuclear and renewable energies: hydropower, wind, solar, bioenergy, geothermal and wave and tidal. 5 Figure 1.1 Global primary energy consumption by source. Note: ‘Other renewable resources’ include geothermal, biomass and waste energy. 5,30 At present, oil is still the largest primary fuel with a share of more than one third in the global primary energy mix and more than 95% of transport energy demand. 31 The World Energy Assessment Report estimates that the future energetical demand can be covered by the total global fossil reserves (i.e. 90% confidence) including oil and gas 33 reserves (i.e. natural gas and methane clathrates); coal, shales and tar sands. Hence, the current sources could support the energy consumption rate globally for at least several centuries. 4 However, due to the increased demand from developing countries the oil cost has increased substantially in the past few years, the amount of imported petroleum is likely to rise to 60% by 2025 in US. 32 On the other hand, there is a high geographical concentration of oil with a growing dependency on a few countries, often politically unstable. Therefore, a mounting anxiety about the economic and geopolitical implications of possible shortages in the supply of oil are expected in the future. Moreover, considering that the current sources would provide an economically viable panorama, the consumption of fossil energy at that rate, will produce a potentially significant global issue in terms of energy supply and climate change. Climate change establishes the correlation between the Earth’s temperature and greenhouse gas (GHG) emissions. The average annual temperature of the Earth has increased since 1800, and the present temperature of Earth is higher than any in thousands of years. 33 Most scientists agree that global warming is attributable to human activities that may have overtaken natural variations as the principal factor influencing the temperature. Proof of this is the production of energy through burning of fossil fuels which constitutes around three-quarters of global greenhouse gas (GHG) emissions. 5 Among these gases, CO2 is the single most important climate-relevant GHG in Earth’s atmosphere, followed by ozone, N2O, CH4, and chlorofluorocarbons. Over a timescale of 10 to 30 years, emitted CO2 accumulates in the atmosphere (~50%) and the near-surface layer of the oceans. 34 This is because CO2 does not condensate and precipitate from the atmosphere by any natural mechanism, unlike water vapor. 35 It has been reported that CO2 will persist globally for the next 500 to 2000 years or more. 36 During the past 650,000 years the atmospheric CO2 concentration has been highly correlated, but is not necessarily the cause, to temperature swings that have caused ice ages on the planet. Over this period, the CO2 concentration has variated between 210 and 300 ppm. 37 In 2010, the CO2 concentration was 390 ppm, which is far in excess of the 280 ppm that is more typical for the interglacial maximum. Considering the scenario predicted in Table 1.1 with an increase of 67.5% of carbon emission rate for 2050 (~650 ppm of CO2 concentration), a major concern rises about environmental effects. With regard to the impacts of climatic change, there will be an increasing number of negative impacts as greater climatic change occurs. These modifications will depend on concurrent changes in the amount, timing, and nature of seasonal precipitation; and 34 also on the extent to which previous human disturbances increase the vulnerability of natural ecosystems to climatic change, to mention a few factors. 6 Despite these uncertainties, given current knowledge, geophysical impacts, biophysical impacts, and impacts on human health and wellbeing, are predicted. Furthermore, it has been stated that at a change in the global mean temperature between 1 and 2 K can lead to trespass the threshold for significant rise of the sea level, and harms in the socio-economic and food-producing systems, and terrestrial ecosystems and forests. 40 Hence, security of supply and climate change represent two major concerns about the future of the energy sector. Urging the need for technology development towards a carbon-neutral power and the intervention of mitigation policies. 41 Regarding technological development, three prominent options for sustainable production of carbonfree energy actively discussed in the literature are: – carbon sequestration, – nuclear energy systems, and – renewable energy resources The challenge for carbon sequestration is finding secure storage for the 25 billion metric tons of CO2 produced annually on Earth. This yearly global emission would occupy a huge volume of 12,500 km3 . Additionally, beyond finding storage volume, carbon sequestration also must prevent leakage. A 1% leak rate would nullify the sequestration effort in a century. Nuclear power, even though has been a key source of low-carbon energy for many countries across the world, relies on finite resources, for instance the terrestrial uranium resource base would be exhausted in 10 years. 42 Moreover, nuclear waste end products constitute an irreversible cycle. 43 On the other side, renewable resources (i.e., wind, hydropower, solar, wind, geothermal, biomass and tidal energy) remain the best option to produce carbon-neutral power. In 2019, around 11% of global primary energy came from renewable technologies, where hydropower represented 60.25% of the energy generated. 30 However, within the various renewable technologies, solar energy is by far the most available resource. More energy from sunlight strikes the earth in 1 h (4.3 x 1020 J) than all of the energy currently consumed on the planet in 1 year (4.1 x 1020 J in 2001). 1 In the next section the advantages and limitations of solar energy will be exposed briefly, important aspects correlating light intermittency to the different types of solar technologies will be discussed in terms of the technology principle and feasibility.
Solar energy
The sun is a major source of inexhaustible free energy for the planet Earth. The solar energy concept consists in the harvesting and utilization of light and/or heat energy generated by the Sun and technologies involved in achieving such goals. 7.6 PW in solar power is incident on the desert regions around the world, and if only tap 10% of it at an energy conversion efficiency of 10%, it would be possible to generate five times the 35 current world’s energy requirement. 43 Figure 1.2 depicts the annual average intensity of solar radiation over the surface of the earth. Research has shown that “black dot” areas could provide more than the entire world’s total primary energy demand, assuming a conversion efficiency of 8%. 44 Despite this huge potential and increase in awareness, the contribution of solar energy to the global energy supply is still negligible, in 2019 only 1.04% of the global primary energy consumption was solar. 30 Indicating that the solar technologies are not yet mature enough to meet the energy demand. Figure 1.2 Annual average solar irradiance distribution over the surface of the Earth. Retrieved from 44 . Solar energy conversion systems fall into three categories according to their primary energy product: (1) solar thermal systems, (2) solar electricity (photovoltaic technology), and (3) solar fuels. Solar thermal energy systems absorb solar irradiation by a solar collector as heat which is then transferred to a working fluid. The heat carried by the working fluid can be used to either provide domestic hot water/heating, which can further be stored.Thermal energy can also be used for electricity generation by the transmission of energy from the working fluid to a generator. Nevertheless, this technology requires a complex design with big areas and the use of materials economically competitive. 45 Photovoltaic technology is based on the principle of solar-to-electric power conversion. In recent years, photovoltaic technology involving the use of semiconductors has become a highly attractive option to address the energy demand. The intense research efforts of scientists regarding solar options have helped to yield an improved efficiency of photovoltaic technology. A triple-junction GaInP/(Al)GaAs/Si device overlying III–V cell combination reported a combined efficiency of 34.5%; and in the case of hybrid perovskite cells, an efficiency increase of ca. 25.5% was reported in 2020 46,47 . The technology is also available on the market, yet, the systems involved the use traditional crystalline silicon or gallium arsenide, commercial thin-film cells (CdTe, amorphous silicon, CuInSe2). Constant progress is done to use thin-film technologies composed of perovskites, organic materials and quantum dots 48 . One of the drawbacks of thermal and electrical solar systems is that the generated energy cannot be directly used as fuel. This factor is also important considering the intermittency of sunlight and its intensity fluctuations. Solar irradiation depends on geographical position, day, time, and season; 49 meaning that once the fuel is produced is necessary to facilitate its distribution and storage. Thermal devices only allow to store heat, which cannot be transformed easily to electricity. Compared to photovoltaic devices and thermal technologies, solar fuels allow the production of a gas that can be readily stored. This overcomes the drawbacks of intermittent solar radiation (inherent day-night and sunny-cloudy cycles), by storing the produced gas during day hours and allowing its posterior dispatchment and distribution. In this way, solar fuels are capable of replacing electricity, by supplying the energy for transport, space conditioning, fabrication processes, cooking, lighting, and communication. 8 Solar fuels technologies involve the production of a gas by means of photocatalysis, and these can be further boosted by means of photoelectrocatalysis. A number of potentially important reactions can be driven by solar light to produce fuels. The two most common photocatalytic reactions are CO2 reduction, to produce fuels such as CO, formate, methane and methanol, ethanol and ethane; and water splitting, to produce H2. 50,51 Figure 1.3 depicts these and other common reactions of solar fuels. These reactions involve multiple electron and proton transfer steps, and they can require electrocatalysts for efficient energy conversion, this is the case for CO2 reduction. Some inroads to CO2 reduction have been made on photo- and electro- catalytic fronts, but generally the precise path to CO2 reduction requires high temperatures ranging from 300 to 400 °C and hence, additional CO2 is generated for producing the energy required to reach such temperatures. 42,52 In addition, the voltage supply for CO2 formation is very negative in water and in most common solvents; consequently, it is necessary a high overpotential for the reaction to occur. 53 On the other hand, hydrogen can be readily produced from water and its reduction can take place at ambient temperatures. Powerful developments have been reported in the last years on artificial nanoscale assemblies of new organic and inorganic materials and morphologies. New methods of nanoscale fabrication, characterization, and simulation create new opportunities for understanding and manipulating the molecular and electronic pathways of solar energy conversion. However, while these laboratory successes demonstrate the appealing promise of direct solar fuel production, there is an enormous gap between the present state of the art and a deployable technology. In the next section 37 a summary about Hydrogen energy is provided, a brief discussion on the hydrogen economy is depicted as well as the means to produce it. Figure 1.3 Scheme of fuels produced by solar energy.
Hydrogen
Nowadays, hydrogen is primarily used in the chemical industry, but its carbon-free characteristics represent a highly attractive solution. Molecular hydrogen is a fuel that does not produce carbon dioxide, it exhibits the highest specific enthalpy of combustion of any chemical fuel, 12 and it can be obtained from renewable sources such as water as will be explained later. From the market perspective, hydrogen is both an energy carrier and a chemical product involved in a range of industrial processes. Overall, hydrogen can be produced from any energy source, whether fossil, nuclear, or renewable. In this respect, the production of hydrogen through low-cost environmentally clean processes by means of renewable energy sources, 54 appears to be a promising pathway to enabling longer-term storage of renewable power and decarbonizing industry and transportation. 10 In this section, the present applications of hydrogen will be described, as well as a brief overview of the production technologies and an introduction into the so-called hydrogen economy.
Hydrogen uses and present demand
The utilization of hydrogen is highly essential as a reactant in industry chemical processes, and in a small scale as a chemical energy. In 2020 hydrogen demand was ~90 Mt (million metric tons), with more than 70 Mt used as pure hydrogen and less than 20 Mt mixed with carbon-containing gases in methanol production and steel manufacturing. Almost all this demand was for its use in oil refining, followed by ammonia and methanol production. 9 Table 1.2 summarizes the main applications of hydrogen by industry sector. Oil refining is the largest consumer of hydrogen today (close to 40 Mt in 2020), hydrogen is used as a reactant in process such as hydrocraking, where it reacts with a hydrocarbon in the presence of a catalyst, for petroleum production. And for ammonia, it is used to hydrogenate sulfur and nitrogen compounds in hydro-processing of ammonia. In the metallurgical and nuclear industry, hydrogen is used to scavenge the oxygen level 38 in boiling water reactors. Another use of hydrogen as direct energy is applied by the NASA as propellant fuel in a mixture of H2 and O2. 55 One of the most attractive commercial uses of hydrogen, is for power generation through fuel cells, which commenced in the early 1990s. A fuel cell engine is an electrochemical cell that splits the cation and the anion in the reactant produce direct current (DC) power to run electric vehicles. Since its powered with hydrogen, the product of the chemical reaction is water. 56 Thanks to this a wide variety of fuel cell vehicles (FCVs) are now commercialized (see Table 1.2).
Chapter 1 State of the Art, problematics and objectives |