Liposomes are vesicles made of lipids. Lipids are organized in lipid bilayers forming sphere shape structures that can encapsulate multiple substances. This property makes liposomes suitable for drug delivery applications. Along 50+ years in liposome research, various preparation methods have been proposed; however, the vast majority of them are batch-based, and mostly production rates are only suitable for laboratory scale production. Moreover, reproducibility is a significant concern required to meet the standards of regulatory agencies. Batch methods do not have the required consistency. These problems delay or block the research, development, and production of liposomes and prevent them from reaching patients worldwide (Hua, de Matos, Metselaar, & Storm, 2018).
Between 1995 and 2005, significant advances were made in microfabrication, especially in soft lithography allowing researchers to produce microfluidic devices with channels in the order of micrometers in relatively easy few steps. These advancements opened an entirely new world of opportunities to investigate the micromixing phenomenon and its applications.
Micromixers offer a suitable alternative for liposome production. Tunable size monodispersed liposome nanoparticles (LNPs) can be produced in a continuous flow, improving reproducibility. Unfortunately, micromixers yield, in general, is too low for industrial-scale production, while the ones that are suitable for mass production clog and are challenging to fabricate. Other mixing strategies and micromixers designs might help to solve this conundrum. However, only a few micromixers have been studied and explicitly characterized for liposome production; furthermore, important variable interactions have not been addressed. This work objective is to investigate liposome formation in micromixers that are based on Dean’s flow dynamics. This type of micromixers uses chaotic advection and Taylor dispersion to accelerate the mixing process. The conditions under which LNPs are produced, as well as crucial factors that direct and control liposome physicochemical characteristics such as size, size distribution, and zeta potential were investigated.
Pharmacy word originates from the Greek word pharmakon, which has different meanings, including remedy and poison. For years, this dichotomy has been accepted. This acceptance meant for patients that sometimes the cure was worse than the disease. Several therapeutic agents must be administered at high doses, increasing toxic effects. For patients, removing or reducing the poisonous part of the remedy would mean a better quality of life. Having a way of releasing the correct dose at the right location without producing side effects, the magic bullet of Paul Ehrlich was an elusive feat until recently (Ehrlich, 1913).
Delivery systems (DS) consists of a shell or structure that functions as a carrier and a cargo that can include genes, drugs, imaging labels, among others. This cargo could be enclosed inside the transport vehicle as well as ligated to its exterior. Most of the DS used in the biomedical field are in the range of 3-200 nm. DS protect their cargo from degradation, offering an extended circulation time inside the human body. Due to their properties such as size and modified surfaces added with specific moieties, DS can target specific organs, tissues, and cells. DS that are in the range of nanometers are called nanoparticle delivery systems (NDS). There are several types of nanoparticles, such as polymeric conjugates, micelles, dendrimers, viral nanoparticles, carbon nanotubes, and liposomes (Cho, Wang, Nie, Chen, & Shin, 2008).
Liposomes
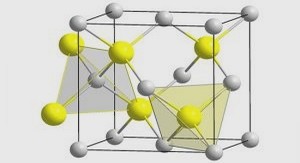
In general, liposomes can be described as sphere-shaped vesicles structured in one or more lipid bilayers. Liposomes are formed by amphiphilic molecules called lipids, composed of a hydrophilic head and two hydrophobic tails. These amphiphilic molecules are insoluble in water except when they are assembled in lipid bilayers with polar heads of the outer layer pointing outwards and the polar heads of the inner layer pointing inwards. This configuration leaves space inside the inner bilayer for hydrophilic formulations and between the bilayers for hydrophobic formulations (Pattni et al., 2015).
The lipids are commonly formed by two hydrocarbon chains esterified to a glycerol backbone or constituted from a ceramide. The hydrophobic part is connected to a polar head that might contain phosphate (“phospholipids”) or carbohydrate units (“glycolipids”).
There are head groups of the amphiphilic molecules that define the surface properties of liposomes formed from them. For instance, the phosphatidylcholine (PC) produces zwitterionic liposomes (Ulrich, 2002). On the other hand, positively charged lipids such as Dioleoyl-3-trimethylammonium propane (DOTAP) are used to create lipoplexes (Tiago Albertini Balbino, Azzoni, & de la Torre, 2013). These liposomes are capable of condensing DNA due to electrostatic interactions and interact with biological membranes. They are used to transfect cells.
Another example is the PEGlylated liposomes, also known as stealth liposomes. The Polyethylene Glycol (PEG) in the head groups of the lipids masks them against opsonization and further destruction by the Mononuclear Phagocyte System (MPS). In this way, liposomes have longer circulation times inside the human body (Allen & Cullis, 2013).
Classification of liposomes
Liposomes can contain multiple bilayers; they are called multilamellar vesicles (MLV) or one single bilayer called unilamellar vesicles. Similarly, classification regarding size is accepted, such as large unilamellar vesicles (LUV), small unilamellar vesicles (SUV), and giant unilamellar vesicles (GUV) (van Swaay & deMello, 2013). Additionally, liposomes classification can also follow function or composition.
Liposomes applications
The capability of liposomes to encapsulate multiple cargos makes them versatile delivery systems.
Drug delivery systems are one of the most prolific fields for liposomes applications. Since the beginning of the field, liposomes were viewed as potential carrier candidates for drugs (Gregoriadis & Ryman, 1971). The first-ever approved nanodrug approved by the FDA was a liposomal formulation composed of cholesterol and a lipid called phosphatidylcholine. It encapsulates Doxorubicin®, a chemotherapy that interferes with the DNA function of rapidly dividing cells (Barenholz, 2012). As opposed to its free form, the liposomal formulation Doxil® showed to accumulate in tumors due to the EPR, reducing side effects (Gabizon et al., 1994). Later, PEGylated liposomes helped to improve circulation time (Safra et al., 2000). PEGylation helps to avoid opsonization and further elimination by Mononuclear Phagocyte System (MPS). By contrast, in some cases, where the disease is affecting precisely the MPS such as leishmaniasis, PEGylation is not necessary because MPS uptake is desirable (New, Chance, Thomas, & Peters, 1978). Currently, liposomes drug delivery applications extend beyond cancer, in antibiotics (Campardelli, Trucillo, & Reverchon, 2018), vaccines (Kanra et al., 2004), and antiviral applications (Croci et al., 2016). New applications include particles that encapsulate two different drugs in one carrier, opening the door to custom therapies (Joshi et al., 2016; Ma, Kohli, & Smith, 2013). Several liposome-based medications have developed and approved by regulatory agencies.
INTRODUCTION |