Hydrogen as an energy carrier
Our society is implementing more and more renewable energy sources such as wind energy, solar energy, etc. For solar and wind energies, the production is usually not in phase with the utilization, so’ a means to store and carry energy is needed: Hydrogen is a renewable energy carrier and could also be used as an energy storage [1 , 2]. Its high gravimetric storage capacity attracted the attention of the researchers and increased their interest in hydrogen fuel and storage technologies.
Hydrogen doesn’t exist freely in nature, it should be released from chemical compounds like hydrocarbons and water. There are many methods to pro duce hydrogen such as steam reforming of natural gas, gasification of coal and biomass, partial oxidation of hydrocarbons and electrolysis ofwater [3] .. In the ideal hydrogen fuel cycle (from water to water , hydrogen is produced by splitting water via electrolysis using solar energy and is stored to produce electrical energy.
Since the by-product of hydrogen reaction in the fuel cell is water, it is environmentally friendlyand can be adopted to secure a clean carbon-free energy for the future [5]. From the energy content point ofview, the gravimetric energy density ofhydrogen is 142 kJ/g which is about three times that of gasoline and almost five times higher than that of coal .
However, the volumetrie energy density of hydrogen at atmospheric pressure and room temperature is too low, 1 kg ofhydrogen occupies a volume of Il m³ . Many efforts are being made in order to find new materials with high volumetrie hydrogen storage capacity.
Hydrogen storage systems
The hydrogen storage system for most practical applications should have a high storage capacity, high gravimetric and volumetrie densities, long and stable cycling performance and safe operation.
Hydrogen can be stored using many ways such as: compressed hydrogen gas, liquid hydrogen, systems based on physisorption ofhydrogen and metal hydride tanks.
Compressed hydrogen gas
Hydrogen is stored in its gaseous form at room temperature in cylinders that can withstand pressures up to 800 bars. At that pressure, the hydrogen volumetrie density is 40 kg/m³ but the gravimetric density is low and this lirnits the practical use of compressed hydrogen . cylinders. In addition, handling highly pressurised compressed cylinders implies safety risks [8].
Liquid hydrogen
Here, hydrogen is stored in its liquid form in cryogenie tanks at around -252 oC and at ambient pressure. Liquid hydrogen has a high volumetrie density of 70.8 g/L. Despite the large amount of energy required for liquefaction, this method is being used to transport large quantities of hydrogen. However, hydrogen boil-off and the cost of advanced insulating tanks to maintain very low temperatures coupled with the energy cost to liquefy hydrogen make this method impractical for on-board vehicular application [9].
Systems based on physisorption of hydrogen
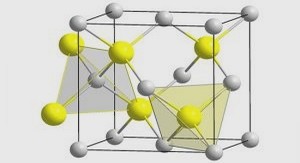
Molecular hydrogen can be adsorbed to the surface of porous materials by the weak Van der Waal forces. Thus, hydrogen molecules can be reversibly stored on high specifie surface area materials such as carbon-based materials and Metal Organic Frameworks (MOFs). The amount ofhydrogen stored at 77 K and under 70 bars on porous carbon and MOFs materials have been found to be around 5 wt % and 7.5 wt% respective1y. Usually, to get significant adsorption, a cryogenie temperature as well as high specifie area and porosity are needed [10, 11].
Introduction |