Télécharger le fichier original (Mémoire de fin d’études)
Data can be divided into 3 regions:
Region 1: high solubility values are obtained by approaching solubility from over–saturated conditions, leading to the formation of a freshly precipitated Zr(OH)4(am) (Deželić, Bilinski, and Wolf 1971; Cho et al. 2005) in equilibrium with polynuclear and colloidal zirconium in solution. The origin of the high solubility values resulting from the strongly over–saturated solutions (Deželić, Bilinski, and Wolf 1971) may be (i) the fast precipitation of amorphous and small particle size of the precipitate and (ii) the slow dehydration and recrystallization kinetics to form a crystalline anhydrous oxide (Brown et al. 2005; Curti and Degueldre 2002).
Region 2: intermediate solubility values are obtained from solubility experiments with initially precipitated Zr(OH)4(am) aged for 3 months (Sasaki et al. 2006). The corresponding aqueous solutions contain monomer, polynuclear and colloidal zirconium. It shall be noticed that the exact stoichiometry of the nominal Zr(OH)4 phase in regions 1 and 2 is not reported. It is an
Chapitre III Etude de la solubilité des nanoparticules de la zircone monoclinique et cubique
oxy–hydroxide of non–determined degree of dehydration of the general formula ZrOx(OH)y(H2O)z . For brevity we keep the custom of calling it Zr(OH)4.
Region 3: low solubility values are measured by approaching solubility from under–saturated conditions using well–crystallized monoclinic ZrO2 (Curti and Degueldre 2002; Kobayashi et al. 2007) and yttrium cubic stabilized ZrO2 (Curti and Degueldre 2002; Pouchon et al. 2001). The two structures are reported to show similar solubility values, leading to suppose that the crystallographic structure may not influence solubility. However there are only few data to confirm this observation and better understanding is necessary.
By analogy to the solubility of tetravalent actinides oxides or hydroxides of U(IV), Th(IV), similar interpretations in the discrepancy of solubility values are discussed when approaching the solubility equilibrium from under–saturated conditions. Altmaier et al. (Altmaier, Neck, and Fanghänel 2004) explain that the variations reside on the presence of a colloidal fraction in solution which can contribute to raise the solubility up to 1.5 – 3 orders of magnitudes. This disparity can be minimized by centrifugation, ultracentrifugation or even ultrafiltration in the same way as for zirconium. The interpretation of actinide solubility data is not straight forward because of the formation of amorphous solids or polynuclear species and colloids which control the solubility at the equilibrium. Altmaier et al., Rai et al. and Neck et al. (Altmaier, Neck, and Fanghänel 2004; Neck et al. 2003) propose a model to explain the solubility of a crystalline AnO2(c) actinide dioxide. At very acid pH, the crystallized AnO2(c) dioxide is thermodynamically stable and constitutes the solid phase limiting the solubility (Rai, Yui, and Moore 2003; Neck et al. 2003). At neutral and alkaline pH, about 6 to 7 orders of magnitude higher experimental values are observed compared to the very low theoretical solubility value of 10−15 mol∙L-1 for well crystalline AnO2(c) (Neck et al. 2003). This has been explained by the authors by the formation of an amorphous surface layer limiting the solubility (Altmaier, Neck, and Fanghänel 2004) in this pH range. Similarly, we may start from the hypothesis that the solubility of tetravalent zirconium is controlled by the crystalline form of ZrO2 at very acid pH. However in contrast to the actinides, between pH 3 and 8 strong differences between amorphous and crystalline phases are observed. Nevertheless, it cannot be excluded that, also in this case, the presence of amorphous surface configurations increases the solubility value between 2 and 8 orders of magnitude compared with the predicted values as low as 10−12 mol∙L-1 (Curti and Degueldre 2002).
The aim of the present paper is to assess zirconia solubility of two crystalline polymorphs varieties, the monoclinic and the yttrium stabilized cubic zirconia phases, using high resolution
Etude de la solubilité des nanoparticules de la zircone monoclinique et cubique
ICP-MS for the determination of the very low concentrations of zirconium. The purpose is also to assess the surface reactivity of the solid since the reversibility cannot be studied by comparing results of approaching solubility from under and over-saturation due to the different phases.
Finally, the solubility constant (log Ks,0(298 K)) obtained at solubility equilibrium on crystalline zirconium oxide will be discussed and compared with solubility constant values of amorphous and hydroxide zirconium.
Experimental
Chemicals
Zirconium (IV) oxide and yttria stabilized zirconium (IV) oxide-yttria stabilized nanopowder were obtained from Sigma-Aldrich. Sodium perchlorate, sodium chloride, hydrochloric acid, perchloric acid, hydrofluoric acid, and nitric acid are ultra-pure chemicals. For further purification from traces of zirconium, hydrochloric and nitric acids were redistilled. All solutions were prepared with ultrapure water from a water purification apparatus (Milli-Q-academic, Millipore).
Batch dissolution experiments
Batch dissolution experiments were performed in Teflon reactor vessels with 120 mL of 0.01 mol∙L-1 NaCl and 0.01 mol∙L-1 NaClO4 solutions. The reactors were cleaned by several washings with HNO3, HCl and Milli-Q water at 100 °C to avoid the release of Zr traces from material prior to use. The pH was adjusted to either 0, 1, 1.5 or 2 by adding respectively Zr free concentrated HCl and HClO4 solutions to 0.01 mol∙L-1 NaCl and 0.01 mol∙L-1 NaClO4 solutions. A combined pH electrode (pHC3006 Ag/AgCl, radiometer) was used to measure the experimental pH in NaCl or NaClO4 solutions (I = 0.01 mol∙L-1) and to control the evolution of the pH with time. No significant deviation from the initial pH was observed over time. The pH was constant ± 0.1.
The reactors were continuously shaken by an orbital stirrer at room temperature and under atmospheric conditions. Solubility experiments were performed from undersaturated conditions with various ratio “nanoparticles surface area/solution volume”, s/v between 104 m-1 and 2×106 m-1.
Chapitre III Etude de la solubilité des nanoparticules de la zircone monoclinique et cubique
Solid phase analytical techniques
Specific surface area (BET). The specific surface area of monoclinic and yttrium stabilized cubic zirconia samples were determined from N2 adsorption isotherms at 77 K obtained with a Micromeritics ASAP 2010 M. Prior to analyses, the samples were outgassed in vacuum overnight. Surface areas for monoclinic and yttrium stabilized cubic zirconia are (36.2 ± 0.1) m²/g and (139.4 ± 0.5) m²/g respectively.
Laser Induced Breakdown Detection (LIBD). A pulsed laser beam (10 Hz, λ = 532 nm) generates plasma events (dielectric breakdowns) selectively on particles in liquid media. The energy impulsion was about 10 mJ with a duration of 6 ns. The number of plasma events per number of total laser pulses and their spatial distribution in the laser focus could give the colloid concentrations and the size particles. The detection limit is 5 ng.L-1 or 105 particles.mL-1 for 10 nm colloids. In this work, this technique was used in the aim to determine the presence of nanoparticles in solution, compared with a solution (ultra-pure deionized water, 18 MΩ/cm, Millipore) free of zirconia particles.
Acid–base titration. Suspension of 27.6 g/L (s/v= 106 m -1) of monoclinic zirconia and 6.6 g/L of cubic zirconia (s/v= 106 m-1) prepared in 10−2 mol/L NaCl at pH 0 were used for the acid-base titration. The suspensions were titrated with 5 mol.L-1 NaOH solution between pH 0 and 2, then with 10−1 mol/L NaOH solution until pH 12. Prior to the experiments, the suspensions were equilibrated for several months. The titrating solution was added by a titrimeter (Titrino DMS 716, Metrohm) by incremental volumes until a final pH of 12 was obtained. After each addition, the pH was measured every 60 seconds by a combined glass electrode, connected to a computer and recorded with Tiamo software program (TiamoTM1.2. – titration and more). The establishment of the equilibrium was assumed if a given reading does not differ for more than 1 mV.min-1 unit. Additional potentiometric titration was performed with the background electrolyte solution alone (free of solid) with identical experimental conditions. The surface site concentration of the dispersed zirconia was determined by using Gran’s function (Gran 1952).The surface sites of density were calculated during the backward titration from pH 2 to pH 12 by addition of NaOH.
Table des matières
Introduction
Chapitre I : Généralités
I. Le zirconium
II. La zircone
II.1. Les phases de la zircone
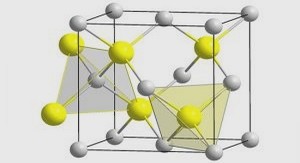
II.1.a. Structure cristalline de la zircone
II.1.b. Stabilisation de la zircone
II.2. Production et formation de la zircone
III. Dissolution de la zircone
III.1. Théories de la dissolution
III.1.a. Théorie de l’état de transition
III.1.b. Théorie basée sur la chimie de coordination
III.1.c. Théorie basée sur la combinaison des approches
III.1.d. Approche expérimentale de Lasaga
III.1.e. Mécanismes à l’interface du système oxyde ou hydroxyde métallique/solution
III.1.f. Notions de la solubilité.
III.2. Solubilité de ZrO2
III.2.a. Equations de solubilité et les constantes thermodynamiques associées
III.2.b. Relation entre solubilité, énergie interfaciale et taille des particules
III.2.c. Résultats de solubilité de ZrO2
IV. Conclusion
Chapitre II : Matériels et méthodes
I. Méthodes expérimentales
I.1. Mise en place des expériences de dissolution de ZrO2
I.1.a. Préparation des échantillons
I.1.b. Mesure de la solubilité
I.2. Les méthodes de caractérisation des solutions
I.2.a. Laser Induced Breakdown Detection (LIBD)
I.2.b. Spectrométrie de masse couplée à un plasma inductif (ICP-MS)
I.2.c. Détermination des impuretés
I.3. Les méthodes de caractérisation des solides
I.3.a. Surface spécifique (BET)
I.3.b. Diffraction des rayons X (DRX)
I.3.c. Diffusion des rayons X aux petits angles (SAXS)
I.3.d. Microscopie électronique en transmission (TEM) et tomographie électronique
I.3.e. Titration potentiométrique
II. Méthodes théoriques
II.1. Equation de Schrödinger
II.2. La théorie de la fonctionnelle de la densité (DFT)
II.2.a. Théorèmes de Hohenberg et Kohn
II.2.b. Méthode de Kohn-Sham
II.2.c. Les fonctionnelles d’échange-corrélation
II.2.d. Notion d’onde plane
II.3. Le code VASP
II.4. Méthodologie
Chapitre III : Etude de la solubilité des nanoparticules de la zircone monoclinique et cubique
Résumé
I. Introduction
II. Experimental
II.1. Chemicals
II.2. Batch dissolution experiments
II.3. Solid phase analytical techniques
II.4. Techniques for solution analyzes
II.4.a. Solubility measurements
II.4.b. Analytical procedure for quantitative analysis of trace levels of zirconium
III. Results and Discussion
III.1. Assessment of the ultrafiltration efficiency
III.2. Solubility of zirconia
III.3. Determination of surface sites fraction involved in the dissolution process
III.4. Solubility constants
IV. Conclusions
V. Acknowledgement
Chapitre IV : Etude de la réactivité de surface des matériaux étudiés
I. Introduction
II. Caractérisation des matériaux avant les expériences d’altération
II.1. Détermination de la surface spécifique par BET
II.2. Caractérisation cristallographique par DRX
II.3. Détermination des impuretés
II.4. Caractérisation par TEM
III. Etude de la réactivité de surfaces des matériaux au cours de la dissolution et à l’équilibre thermodynamique
III.1. Evolution de la morphologie par SAXS
III.2. Caractérisation par DRX capillaire
III.3. Etude morphologique des nanoparticules après altération par HR-TEM et STEM
III.5. Effet de la taille des particules sur la solubilité de ZrO2
III.6. Réactivité de la surface de ZrO2
IV. Conclusion
Chapitre V : Compréhension des mécanismes de la faible dissolution du dioxyde de zirconium
I. Introduction
II. Bulk
II.1. Structure
II.2. Paramètres de maille
III. Surfaces stoechiométriques parfaites [111] et [101] de la zircone monoclinique
III.1. Les supercellules
III.2. Structure des deux terminaisons considérées [111] et [101]
III.3. Réactivité des deux surfaces parfaites [111] et [101]
IV. Surfaces stoechiométriques à marches [111] et [101] de la zircone monoclinique
IV.1. Structure des deux terminaisons à marches [111] et [101]
IV.2. Réactivité des deux terminaisons à marches [111] et [101]
IV.2.a. Adsorption de l’eau
IV.2.b. Hydratation des surfaces à marches [111] et [101]
IV.2.c. Adsorption de Zr(OH)4
V. Conclusion
Conclusions générales et perspectives.
Références bibliographiques
Annexes
I. Titration potentiométrique
II. Théorie de la fonctionnelle de la densité (DFT)